Page 60 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 60
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
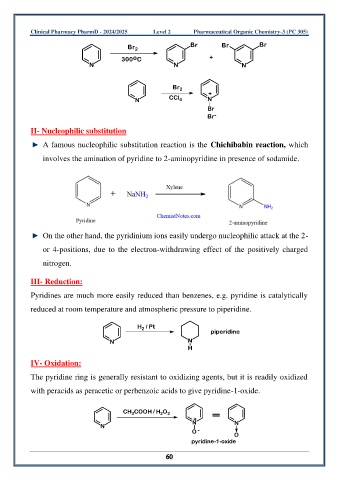
Br 2 Br Br Br
o
300 C +
N N N
Br 2
+
N CCl 4 N
Br
Br -
II- Nucleophilic substitution
A famous nucleophilic substitution reaction is the Chichibabin reaction, which
involves the amination of pyridine to 2-aminopyridine in presence of sodamide.
On the other hand, the pyridinium ions easily undergo nucleophilic attack at the 2-
or 4-positions, due to the electron-withdrawing effect of the positively charged
nitrogen.
III- Reduction:
Pyridines are much more easily reduced than benzenes, e.g. pyridine is catalytically
reduced at room temperature and atmospheric pressure to piperidine.
H / Pt
2
piperidine
N N
H
IV- Oxidation:
The pyridine ring is generally resistant to oxidizing agents, but it is readily oxidized
with peracids as peracetic or perbenzoic acids to give pyridine-1-oxide.
CH COOH / H O 2
3
2
+
N N
N
O -
O
pyridine-1-oxide