Page 59 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 59
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
B) Substitution at the carbon:
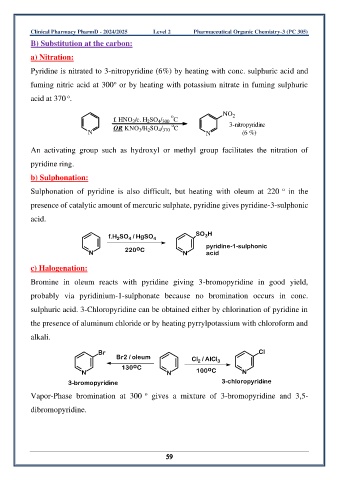
a) Nitration:
Pyridine is nitrated to 3-nitropyridine (6%) by heating with conc. sulphuric acid and
o
fuming nitric acid at 300 or by heating with potassium nitrate in fuming sulphuric
o
acid at 370 .
NO 2
o
f. HNO /c. H SO / C
4 300
2
3
o
OR KNO /H SO / C 3-nitropyridine
N 3 2 4 370 N (6 %)
An activating group such as hydroxyl or methyl group facilitates the nitration of
pyridine ring.
b) Sulphonation:
o
Sulphonation of pyridine is also difficult, but heating with oleum at 220 in the
presence of catalytic amount of mercuric sulphate, pyridine gives pyridine-3-sulphonic
acid.
f.H SO / HgSO 4 SO H
3
2
4
o
acid
N 220 C N pyridine-1-sulphonic
c) Halogenation:
Bromine in oleum reacts with pyridine giving 3-bromopyridine in good yield,
probably via pyridinium-1-sulphonate because no bromination occurs in conc.
sulphuric acid. 3-Chloropyridine can be obtained either by chlorination of pyridine in
the presence of aluminum chloride or by heating pyrrylpotassium with chloroform and
alkali.
Br Cl
Br2 / oleum Cl / AlCl 3
2
o
130 C o
N N 100 C N
3-bromopyridine 3-chloropyridine
Vapor-Phase bromination at 300 gives a mixture of 3-bromopyridine and 3,5-
o
dibromopyridine.