Page 19 - MSC手冊 20220831
P. 19
RA
Dovepress Wang et al 類風濕性關節炎
臍帶間質幹細胞(MSC)治療類風濕性關節炎第I/II期人體臨床試驗,
經過3年治療,雙手已可自由伸展
Drug Design, Development and Therapy Dovepress
open access to scientific and medical research
Open Access Full Text Article ORIGINAL RE SE ARCH
Efficacy and Safety of Umbilical Cord
Dovepress Wang et al
Mesenchymal Stem Cell Therapy for Rheumatoid
Arthritis Patients: A Prospective Phase I/II Study
Figure 5 Scores of DAS28 and HAQ were evaluated after twice of UC-MSCs treatment. (A) DAS28 score was evaluated; (B) HAQ score was evaluated. Pre-treatment
versus after the first or second treatment; *** represents P < 0.001, 1-year posttreatment versus 3-year posttreatment; * represents P < 0.05 (n = 64).
This article was published in the following Dove Press journal:
A Drug Design, Development and Therapy
B
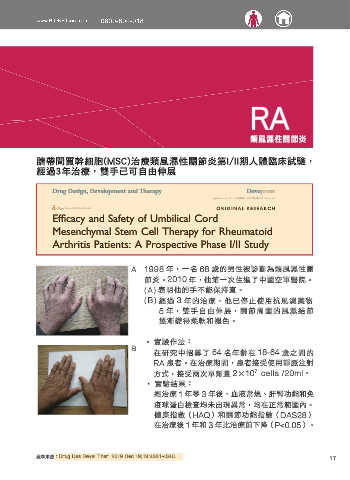
A 1998 年,一名 68 歲的男性被診斷為類風濕性關
節炎。2010 年,他第一次住進了中國空軍醫院。
1,
Liming Wang * Background: The traditional anti-inflammation disease-modifying anti-rheumatic drugs
(A) 表明他的手不能保持直。
Shigao Huang 2, * (DMARDs) have limited therapeutic effects in rheumatoid arthritis (RA) patients. We
Shimei Li 1 previously reported the safety and efficacy of umbilical cord mesenchymal stem cell (UC-
(B) 經過 3 年的治療,他已停止使用抗風濕藥物
Ming Li 1 MSC) treatment in RA patients that were observed for up to 8 months after UC-MSC
Jun Shi 1 infusion. The aim of this study is to assess the long-term efficacy and safety of UC-MSC
5 年, 雙手 自 由 伸展,關 節 周 圍的 風 濕 結節
Wen Bai 1 along with DMARDs for the treatment of RA.
逐漸變得柔軟和褪色。
1 Methods: 64 RA patients aged 18–64 years were recruited in the study. During the
7
Qianyun Wang HAQ score was evaluated. Pre-treatment
Figure 5 Scores of DAS28 and HAQ were evaluated after twice of UC-MSCs treatment. (A) DAS28 score was evaluated; (B) treatment, patients were treated with 40 mL UC-MSC suspension product (2 × 10 cells/
versus after the first or second treatment; *** represents P < 0.001, 1-year posttreatment versus 3-year posttreatment; 3 * represents P < 0.05 (n = 64).
Libo Zheng 20 mL) via intravenous injection immediately after the infusion of 100 mL saline. The
Yongjun Liu 3 serological markers tests were used to assess safety and the 28-joint disease activity score
A Figure 6 A 68 1 year-male was diagnosed with RA in 1998. In 2010, he was admitted to our hospital for the first time. (A) Shows that his hands could not be kept straight. (B)
B
˙ 實驗作法:
Cell Therapy Center, 986 Hospital of
(DAS28) and the Health Assessment Questionnaire (HAQ) to assess efficacy.
After 3 years posttreatment, he has stopped using anti-rheumatism medicine for 5 years, and his hands stretch freely and the rheumatic nodules around the joints gradually
B
People’s Liberation Army Air Force,
become soft and fade. Results: 1 year and 3 years after UC-MSC cells treatment, the blood routine, liver and
Xi’an, Shaanxi, People’s Republic of 在研究中招募了 64 名年齡在 18-64 歲之間的
2
China; Cancer Center, Institute of kidney function and immunoglobulin examination showed no abnormalities, which were all
Translational Medicine, Faculty of Healthrun some-
and swelling was well-healed. In addition, she can in the normal range. The ESR, CRP, RF of 1 year and 3 years after treatment and anti-CCP of
few patients (4%) showed mild adverse effects such as flu-
RA 患者。在治療期間,患者接受使用靜脈注射
Sciences, University of Macau, Taipa,
times freely and do some physical exercise. 3 years after treatment were detected to be lower than that of pretreatment, which showed
like symptoms during the infusion, which disappeared
Macao SAR, People’s Republic of China;
7
方式,接受兩次單劑量 2×10 cells /20ml。
within hours without any treatment. All patients have
3 Stem Cell Biology and Regenerative significant change (P < 0.05). Health index (HAQ) and joint function index (DAS28)
Discussion decreased 1 year and 3 years after treatment than before treatment (P < 0.05).
shown improvements in the diet, sleep, and physical
Medicine Institution, Yi-Chuang Institute
˙ 實驗結果:
strength after the cell therapy based on patients’ reports.
of Bio-Industry, Beijing, People’s Republic UC-MSC
In our previous report, 9,12 we have shown the Conclusion: UC-MSC cells plus DMARDs therapy can be a safe, effective and feasible
of China
treatment was safe and effective in clinical treatment. We In comparison, there was no such improvement in the
therapeutic option for RA patients.
經治療 1 年零 3 年後,血液常規、肝腎功能和免
observed the patients up to 8 months after UC-MSC treat- control group. In addition, the clinical response to UC-
*These authors contributed equally to
Keywords: rheumatoid arthritis, umbilical cord mesenchymal stem cell, cell therapy
ment in RA patients, which no acute serious side-effects MSCs treatment was rapid (as early as 12 h posttreatment)
this work
疫球蛋白檢查均未出現異常,均在正常範圍內。
occurred either during or after UC-MSCs infusion, and only with the physical evidence after the administration of
健康指數(HAQ)和關節功能指數(DAS28)
Introduction
Drug Design, Development and Therapy 2019:13 could not be kept straight. (B)
Figure 6 A 68 year-male was diagnosed with RA in 1998. In 2010, he was admitted to our hospital for the first time. (A) Shows that his hands submit your manuscript | www.dovepress.com 4337
Rheumatoid arthritis (RA) is a chronic, autoimmune disease affecting multiple joints
DovePress
After 3 years posttreatment, he has stopped using anti-rheumatism medicine for 5 years, and his hands stretch freely and the rheumatic nodules around the joints gradually 在治療後 1 年和 3 年比治療前下降(P<0.05)。
become soft and fade. 1
symmetrically. The main symptoms at the early stage of the disease are joint pain and
Correspondence: Shigao Huang swelling; at late stage, arthritis leads to joint stiffness, malformation, loss of function, and
Cancer Center, Institute of Translational such as flu-
and swelling was well-healed. In addition, she can run some- few patients (4%) showed mild adverse effects 2
times freely and do some physical exercise. like symptoms during the infusion, whi even disability. Currently, available medication for RA treatment has limited efficacy,
Medicine, Faculty of Health Sciences,ch disappeared
University of Macau, Room 3013, Building including non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-
within hours without any treatment. All patients have
N-22, Taipa, Macau, People’s Republic of
3
Discussion 資料來源:Drug Des Devel Ther. 2019 Dec 19;13:4331-4340. and immunosuppressants. The target cytokine therapy 17
rheumatic drugs (DMARDs)
shown improvements in the diet, sleep, and physical
China
Email huangshigao2010@aliyun.com patients’ reports.
In our previous report, 9,12 we have shown the UC-MSC strength after the cell therapy based on has some therapeutic effects but without significant repair of injured joints, which
treatment was safe and effective in clinical treatment. We In comparison, there was no such improvement in the
restricts its widespread clinical use.
Yongjun Liu
Stem Cell Biology and Regenerative response to UC-
observed the patients up to 8 months after UC-MSC treat- control group. In addition, the clinical Mesenchymal stem cells (MSCs) are characterized by their regenerative property
Medicine Institution, Yi-Chuang Institute
ment in RA patients, which no acute serious side-effects MSCs treatment was rapid (as early as 12 h posttreatment)
to repair parenchymal tissue and organs through differentiating into lineages of
of Bio-Industry, No. 35, Jinghai 3 Road
occurred either during or after UC-MSCs infusion, and only with the physical evidence after the administration of 4
Economic-Technological Development
Area, Beijing, People’s Republic of China mesenchymal tissues, as well as immunomodulation. Injected MSCs can migrate
5
Email andyliuliu2001@aliyun.com to injured tissues and facilitate the recovery of damaged cells. Clinical studies have
Drug Design, Development and Therapy 2019:13 submit your manuscript | www.dovepress.com 4337
DovePress
submit your manuscript | www.dovepress.com Drug Design, Development and Therapy 2019:13 4331–4340 4331
DovePress © 2019 Wang et al. This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.
php and incorporate the Creative Commons Attribution – Non Commercial (unported, v3.0) License (http://creativecommons.org/licenses/by-nc/3.0/). By accessing the
http://doi.org/10.2147/DDDT.S225613
work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For
permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms (https://www.dovepress.com/terms.php).