Page 113 - phytochemistry general program
P. 113
one of the following phenomena: adsorption; partition; ion exchange; molecular
exclusion; affinity or zone electrophoresis.
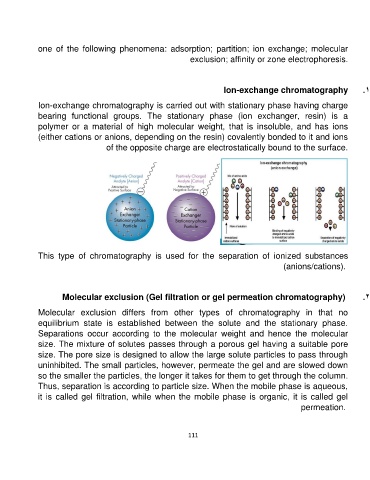
Ion-exchange chromatography .1
Ion-exchange chromatography is carried out with stationary phase having charge
bearing functional groups. The stationary phase (ion exchanger, resin) is a
polymer or a material of high molecular weight, that is insoluble, and has ions
(either cations or anions, depending on the resin) covalently bonded to it and ions
of the opposite charge are electrostatically bound to the surface.
This type of chromatography is used for the separation of ionized substances
(anions/cations).
Molecular exclusion (Gel filtration or gel permeation chromatography) .2
Molecular exclusion differs from other types of chromatography in that no
equilibrium state is established between the solute and the stationary phase.
Separations occur according to the molecular weight and hence the molecular
size. The mixture of solutes passes through a porous gel having a suitable pore
size. The pore size is designed to allow the large solute particles to pass through
uninhibited. The small particles, however, permeate the gel and are slowed down
so the smaller the particles, the longer it takes for them to get through the column.
Thus, separation is according to particle size. When the mobile phase is aqueous,
it is called gel filtration, while when the mobile phase is organic, it is called gel
permeation.
111