Page 116 - phytochemistry general program
P. 116
Adsorption chromatography can be illustrated in techniques such as: thin layer
chromatography, column chromatography [both conventional and high
performance liquid chromatography (HPLC)] and gas-solid chromatography
(GSC).
Effect of solute functional groups on adsorption chromatography
The major factor in adsorption chromatography is the preferential adsorption of
different solutes leading to different extent of adsorption.
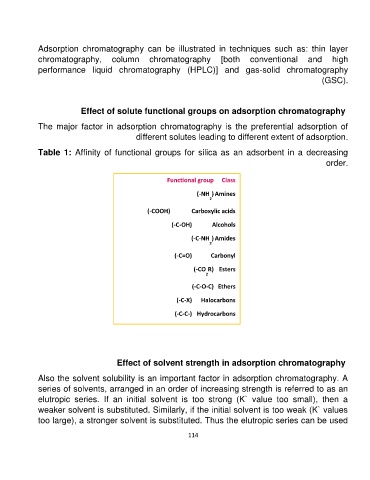
Table 1: Affinity of functional groups for silica as an adsorbent in a decreasing
order.
Functional group Class
(-NH ) Amines
2
(-COOH) Carboxylic acids
(-C-OH) Alcohols
(-C-NH ) Amides
2
(-C=O) Carbonyl
(-CO R) Esters
2
(-C-O-C) Ethers
(-C-X) Halocarbons
(-C-C-) Hydrocarbons
Effect of solvent strength in adsorption chromatography
Also the solvent solubility is an important factor in adsorption chromatography. A
series of solvents, arranged in an order of increasing strength is referred to as an
elutropic series. If an initial solvent is too strong (K` value too small), then a
weaker solvent is substituted. Similarly, if the initial solvent is too weak (K` values
too large), a stronger solvent is substituted. Thus the elutropic series can be used
114