Page 25 - Phytochemistry 2 (06-PG 605)
P. 25
The pair of enantiomers with the stereo chemistry (1R,2S) and (1S,2R) is
designated ephedrine, while the pair of enantiomers with the stereochemistry
(1R,2R) and (1S,2S) is called pseudoephedrine.
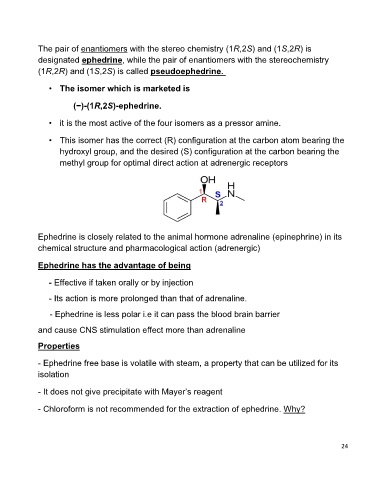
• The isomer which is marketed is
(−)-(1R,2S)-ephedrine.
• it is the most active of the four isomers as a pressor amine.
• This isomer has the correct (R) configuration at the carbon atom bearing the
hydroxyl group, and the desired (S) configuration at the carbon bearing the
methyl group for optimal direct action at adrenergic receptors
Ephedrine is closely related to the animal hormone adrenaline (epinephrine) in its
chemical structure and pharmacological action (adrenergic)
Ephedrine has the advantage of being
- Effective if taken orally or by injection
- Its action is more prolonged than that of adrenaline.
- Ephedrine is less polar i.e it can pass the blood brain barrier
and cause CNS stimulation effect more than adrenaline
Properties
- Ephedrine free base is volatile with steam, a property that can be utilized for its
isolation
- It does not give precipitate with Mayer’s reagent
- Chloroform is not recommended for the extraction of ephedrine. Why?
24