Page 30 - Analytical Chemistry I E-book
P. 30
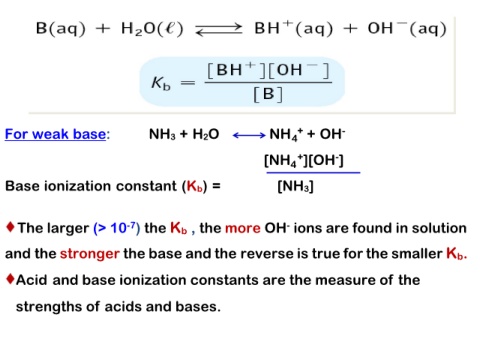
For weak base: NH3 + H2O NH4+ + OH-
[NH4+][OH-]
Base ionization constant (Kb) =
[NH3]
The larger (> 10-7) the Kb , the more OH- ions are found in solution
and the stronger the base and the reverse is true for the smaller Kb.
Acid and base ionization constants are the measure of the
strengths of acids and bases.