Page 29 - Analytical Chemistry I E-book
P. 29
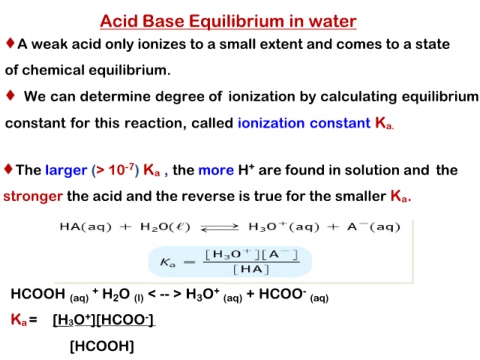
Acid Base Equilibrium in water
A weak acid only ionizes to a small extent and comes to a state
of chemical equilibrium.
We can determine degree of ionization by calculating equilibrium
constant for this reaction, called ionization constant Ka.
The larger (> 10-7) Ka , the more H+ are found in solution and the
stronger the acid and the reverse is true for the smaller Ka.
HCOOH (aq) + H2O (l) < -- > H3O+ (aq) + HCOO- (aq)
Ka = [H3O+][HCOO-]
[HCOOH]