Page 40 - Analytical Chemistry I E-book
P. 40
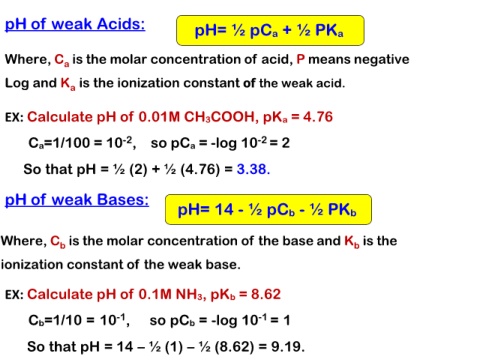
pH of weak Acids: pH= ½ pCa + ½ PKa
Where, Ca is the molar concentration of acid, P means negative
Log and Ka is the ionization constant of the weak acid.
EX: Calculate pH of 0.01M CH3COOH, pKa = 4.76
Ca=1/100 = 10-2, so pCa = -log 10-2 = 2
So that pH = ½ (2) + ½ (4.76) = 3.38.
pH of weak Bases: pH= 14 - ½ pCb - ½ PKb
Where, Cb is the molar concentration of the base and Kb is the
ionization constant of the weak base.
EX: Calculate pH of 0.1M NH3, pKb = 8.62
Cb=1/10 = 10-1, so pCb = -log 10-1 = 1
So that pH = 14 – ½ (1) – ½ (8.62) = 9.19.