Page 42 - Analytical Chemistry I E-book
P. 42
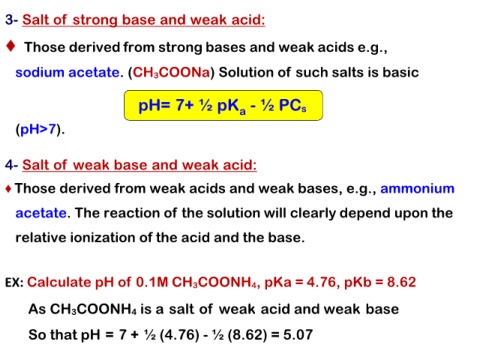
3- Salt of strong base and weak acid:
Those derived from strong bases and weak acids e.g.,
sodium acetate. (CH3COONa) Solution of such salts is basic
pH= 7+ ½ pKa - ½ PCs
(pH>7).
4- Salt of weak base and weak acid:
Those derived from weak acids and weak bases, e.g., ammonium
acetate. The reaction of the solution will clearly depend upon the
relative ionization of the acid and the base.
EX: Calculate pH of 0.1M CH3COONH4, pKa = 4.76, pKb = 8.62
As CH3COONH4 is a salt of weak acid and weak base
So that pH = 7 + ½ (4.76) - ½ (8.62) = 5.07