Page 87 - Analytical Chemistry I E-book
P. 87
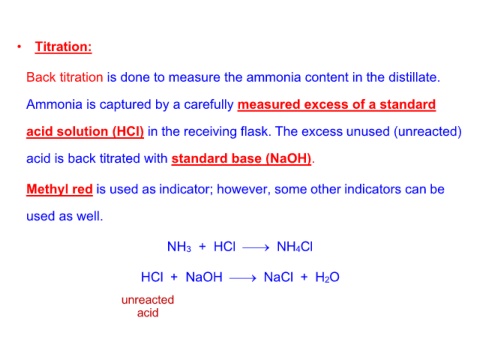
• Titration:
Back titration is done to measure the ammonia content in the distillate.
Ammonia is captured by a carefully measured excess of a standard
acid solution (HCl) in the receiving flask. The excess unused (unreacted)
acid is back titrated with standard base (NaOH).
Methyl red is used as indicator; however, some other indicators can be
used as well.
NH3 + HCl ⎯→ NH4Cl
HCl + NaOH ⎯→ NaCl + H2O
unreacted
acid