Page 7 - Instrumental Analysis - Pharm D Clinical- 07-PA403
P. 7
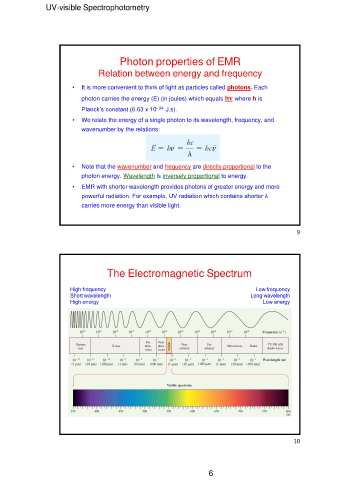
UV-visible Spectrophotometry
Photon properties of EMR
Relation between energy and frequency
• It is more convenient to think of light as particles called photons. Each
photon carries the energy (E) (in joules) which equals hν where h is
Planck’s constant (6.63 x 10–34 J.s).
• We relate the energy of a single photon to its wavelength, frequency, and
wavenumber by the relations:
• Note that the wavenumber and frequency are directly proportional to the
photon energy. Wavelength is inversely proportional to energy.
• EMR with shorter wavelength provides photons of greater energy and more
powerful radiation. For example, UV radiation which contains shorter λ
carries more energy than visible light.
9
The Electromagnetic Spectrum
High frequency Low frequency
Short wavelength Long wavelength
High energy
Low energy
10
6