Page 12 - Instrumental Analysis - Pharm D Clinical- 07-PA403
P. 12
UV-visible Spectrophotometry
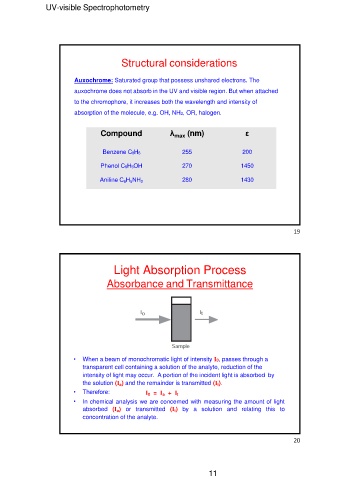
Structural considerations
Auxochrome: Saturated group that possess unshared electrons. The
auxochrome does not absorb in the UV and visible region. But when attached
to the chromophore, it increases both the wavelength and intensity of
absorption of the molecule, e.g. OH, NH2, OR, halogen.
Compound λmax (nm) ε
Benzene C6H6 255 200
Phenol C6H5OH 270 1450
Aniline C6H5NH2 280 1430
19
Light Absorption Process
Absorbance and Transmittance
• When a beam of monochromatic light of intensity I0, passes through a
transparent cell containing a solution of the analyte, reduction of the
intensity of light may occur. A portion of the incident light is absorbed by
the solution (Ia) and the remainder is transmitted (It).
• Therefore: I0 = Ia + It
• In chemical analysis we are concerned with measuring the amount of light
absorbed (Ia) or transmitted (It) by a solution and relating this to
concentration of the analyte.
20
11