Page 15 - Instrumental Analysis - Pharm D Clinical- 07-PA403
P. 15
UV-visible Spectrophotometry
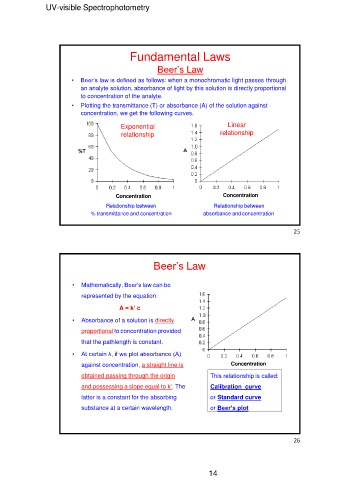
Fundamental Laws
Beer’s Law
• Beer’s law is defined as follows: when a monochromatic light passes through
an analyte solution, absorbance of light by this solution is directly proportional
to concentration of the analyte.
• Plotting the transmittance (T) or absorbance (A) of the solution against
concentration, we get the following curves.
Exponential Linear
relationship relationship
Concentration Concentration
Relationship between Relationship between
% transmittance and concentration absorbance and concentration
25
Beer’s Law
• Mathematically, Beer’s law can be Concentration
represented by the equation:
A = k’ c This relationship is called:
Calibration curve
• Absorbance of a solution is directly or Standard curve
proportional to concentration provided or Beer’s plot
that the pathlength is constant.
• At certain λ, if we plot absorbance (A)
against concentration, a straight line is
obtained passing through the origin
and possessing a slope equal to k’. The
latter is a constant for the absorbing
substance at a certain wavelength.
26
14