Page 14 - Instrumental Analysis - Pharm D Clinical- 07-PA403
P. 14
UV-visible Spectrophotometry
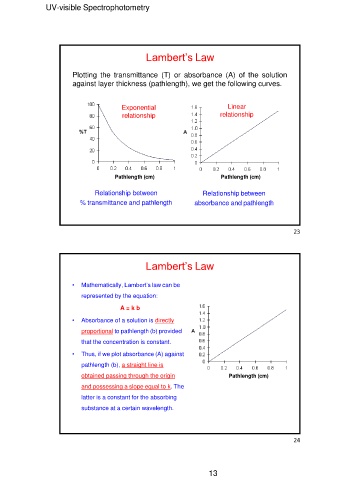
Lambert’s Law
Plotting the transmittance (T) or absorbance (A) of the solution
against layer thickness (pathlength), we get the following curves.
Exponential Linear
relationship relationship
Pathlength (cm) Pathlength (cm)
Relationship between Relationship between
% transmittance and pathlength absorbance and pathlength
23
Lambert’s Law Pathlength (cm)
• Mathematically, Lambert’s law can be
represented by the equation:
A=kb
• Absorbance of a solution is directly
proportional to pathlength (b) provided
that the concentration is constant.
• Thus, if we plot absorbance (A) against
pathlength (b), a straight line is
obtained passing through the origin
and possessing a slope equal to k. The
latter is a constant for the absorbing
substance at a certain wavelength.
24
13