Page 67 - MNU-PM502- Pharmaeutical Microbiology Theoritical Book
P. 67
Pharm D- Clinical Pharmacy Program Third Level Pharmaceutical Microbiology& Antimicrobials (PM 502)
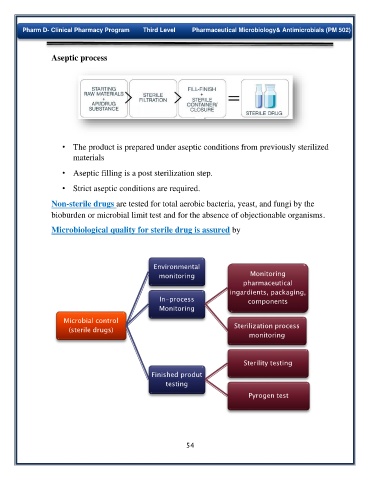
Aseptic process
• The product is prepared under aseptic conditions from previously sterilized
materials
• Aseptic filling is a post sterilization step.
• Strict aseptic conditions are required.
Non-sterile drugs are tested for total aerobic bacteria, yeast, and fungi by the
bioburden or microbial limit test and for the absence of objectionable organisms.
Microbiological quality for sterile drug is assured by
Environmental
monitoring Monitoring
pharmaceutical
ingardients, packaging,
In-process components
Monitoring
Microbial control
(sterile drugs) Sterilization process
monitoring
Sterility testing
Finished produt
testing
Pyrogen test
54