Page 19 - PC 101 Interactive practical book
P. 19
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
Steps for the determination of a sample using
titration
(3 steps to determine the sample concentration)
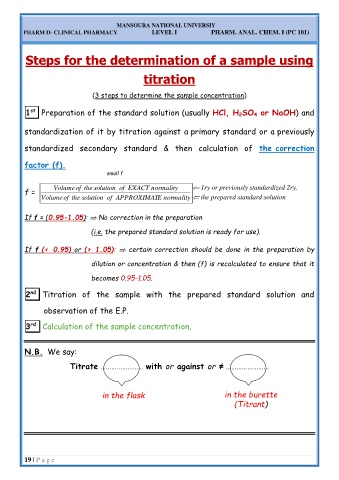
1 st Preparation of the standard solution (usually HCl, H 2SO 4 or NaOH) and
standardization of it by titration against a primary standard or a previously
standardized secondary standard & then calculation of the correction
factor (f).
small f
f = Volume of the solution of EXACT normality 1ry or previously standardized 2ry.
Volume of the solution of APPROXIMAT E normality the prepared standard solution
No correction in the preparation
If f = (0.95-1.05):
(i.e. the prepared standard solution is ready for use).
If f (< 0.95) or (> 1.05): certain correction should be done in the preparation by
dilution or concentration & then (f) is recalculated to ensure that it
becomes 0.95-1.05.
2 nd Titration of the sample with the prepared standard solution and
observation of the E.P.
3 rd Calculation of the sample concentration.
N.B. We say:
Titrate …………………… with or against or ≠ ……………………
in the flask in the burette
(Titrant)
19 | P a g e