Page 29 - PC 101 Interactive practical book
P. 29
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
but without the sample and the E.P. in this case is called
Blank Reading.
Blank determination is required in this experiment because
when NaOH is heated and then cooled, its strength is decreased due to:
1- Interaction of NaOH with the glass of the flask.
2- Absorption of atm. CO 2.
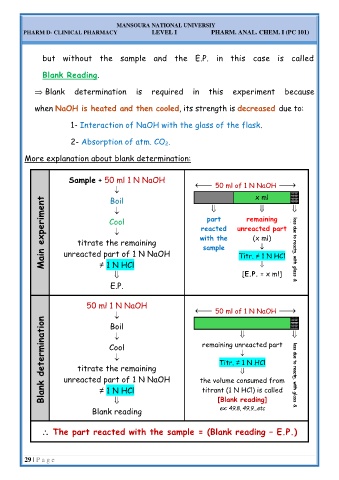
More explanation about blank determination:
Sample + 50 ml 1 N NaOH
50 ml of 1 N NaOH
x ml
Main experiment titrate the remaining with the unreacted part CO 2 loss due to reactn. with glass &
Boil
remaining
part
Cool
reacted
(x ml)
sample
unreacted part of 1 N NaOH
≠ 1 N HCl
[E.P. = x ml]
Titr. ≠ 1 N HCl
E.P.
50 ml 1 N NaOH
50 ml of 1 N NaOH
Blank determination unreacted part of 1 N NaOH the volume consumed from CO 2 loss due to reactn. with glass &
Boil
remaining unreacted part
Cool
Titr. ≠ 1 N HCl
titrate the remaining
≠ 1 N HCl
[Blank reading]
ex: 49.8, 49.9...etc
Blank reading titrant (1 N HCl) is called
The part reacted with the sample = (Blank reading – E.P.)
29 | P a g e