Page 84 - PC 101 Interactive practical book
P. 84
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
Exp.: Determination of KBr by Volhard's method
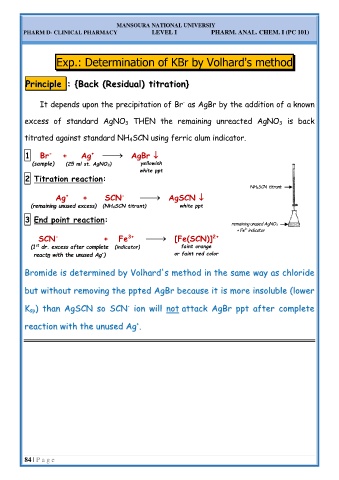
P Pr ri in nc ci ip pl le e : : { {B Ba ac ck k ( (R Re es si id du ua al l) ) t ti it tr ra at ti io on n} }
It depends upon the precipitation of Br as AgBr by the addition of a known
-
excess of standard AgNO 3 THEN the remaining unreacted AgNO 3 is back
titrated against standard NH 4SCN using ferric alum indicator.
+
1 Br + Ag AgBr
-
(sample) (25 ml st. AgNO 3) yellowish
white ppt
2 Titration reaction:
NH 4SCN titrant
Ag + SCN AgSCN
-
+
(remaining unused excess) (NH 4SCN titrant) white ppt
3 End point reaction:
remaining unused AgNO 3
+ Fe indicator
3+
SCN + Fe [Fe(SCN)]
-
2+
3+
st faint orange
(1 dr. excess after complete (indicator)
reactn with the unused Ag ) or faint red color
+
B Br ro om mi id de e i is s d de et te er rm mi in ne ed d b by y V Vo ol lh ha ar rd d' 's s m me et th ho od d i in n t th he e s sa am me e w wa ay y a as s c ch hl lo or ri id de e
b bu ut t w wi it th ho ou ut t r re em mo ov vi in ng g t th he e p pp pt te ed d A Ag gB Br r b be ec ca au us se e i it t i is s m mo or re e i in ns so ol lu ub bl le e ( (l lo ow we er r
K K s sp p) ) t th ha an n A Ag gS SC CN N s so o S SC CN N i io on n w wi il ll l n no ot t a at tt ta ac ck k A Ag gB Br r p pp pt t a af ft te er r c co om mp pl le et te e
- -
r re ea ac ct ti io on n w wi it th h t th he e u un nu us se ed d A Ag g . .
+ +
84 | P a g e