Page 49 - on going bismillah emodul 1 - Copy_Neat
P. 49
34
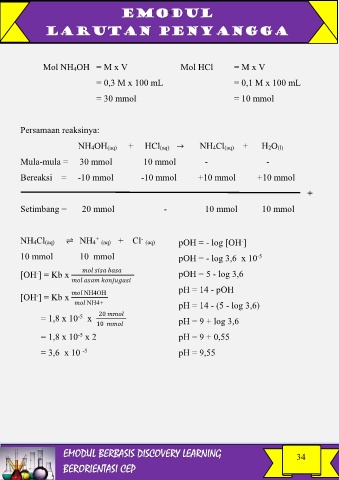
Mol NH4OH = M x V Mol HCl = M x V
= 0,3 M x 100 mL = 0,1 M x 100 mL
= 30 mmol = 10 mmol
Persamaan reaksinya:
NH4OH(aq) + HCl(aq) → NH4Cl(aq) + H2O(l)
Mula-mula = 30 mmol 10 mmol - -
Bereaksi = -10 mmol -10 mmol +10 mmol +10 mmol
+
Setimbang = 20 mmol - 10 mmol 10 mmol
-
+
-
NH4Cl(aq) ⇌ NH4 (aq) + Cl (aq) pOH = - log [OH ]
10 mmol 10 mmol pOH = - log 3,6 x 10 -5
-
[OH ] = Kb x pOH = 5 - log 3,6
pH = 14 - pOH
[OH ] = Kb x NH4OH
-
NH4+ pH = 14 - (5 - log 3,6)
= 1,8 x 10 -5 x 20 pH = 9 + log 3,6
10
-5
= 1,8 x 10 x 2 pH = 9 + 0,55
= 3,6 x 10 -5 pH = 9,55
34