Page 16 - Practical DF2 Corrected (2)
P. 16
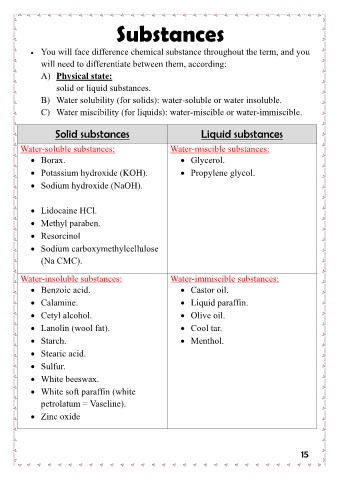
Substances
• You will face difference chemical substance throughout the term, and you
will need to differentiate between them, according:
A) Physical state:
solid or liquid substances.
B) Water solubility (for solids): water-soluble or water insoluble.
C) Water miscibility (for liquids): water-miscible or water-immiscible.
Solid substances Liquid substances
Water-soluble substances: Water-miscible substances:
• Borax. • Glycerol.
• Potassium hydroxide (KOH). • Propylene glycol.
• Sodium hydroxide (NaOH).
• Lidocaine HCl. Water-immiscible substances:
• Methyl paraben. • Castor oil.
• Resorcinol • Liquid paraffin.
• Sodium carboxymethylcellulose • Olive oil.
• Cool tar.
(Na CMC). • Menthol.
Water-insoluble substances:
• Benzoic acid.
• Calamine.
• Cetyl alcohol.
• Lanolin (wool fat).
• Starch.
• Stearic acid.
• Sulfur.
• White beeswax.
• White soft paraffin (white
petrolatum = Vaseline).
• Zinc oxide
15