Page 30 - P4304.1-V102_PS Magazine - Nov 24
P. 30
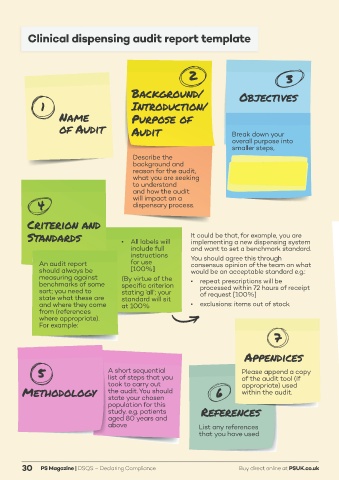
Clinical dispensing audit report template
2 3
Background/ Objectives
1 Introduction/
Name Purpose of
of Audit Audit Break down your
overall purpose into
smaller steps,
Describe the e.g. Ensuring that all
background and medication labels
reason for the audit, include full instructions
what you are seeking for usage rather than
to understand as required etc.
and how the audit
4 will impact on a
dispensary process.
Criterion and
Standards • All labels will It could be that, for example, you are
implementing a new dispensing system
include full and want to set a benchmark standard.
instructions You should agree this through
An audit report for use consensus opinion of the team on what
should always be [100%] would be an acceptable standard e.g.:
measuring against (By virtue of the
benchmarks of some specific criterion • repeat prescriptions will be
sort; you need to stating ‘all’; your processed within 72 hours of receipt
state what these are standard will sit of request [100%]
and where they come at 100% • exclusions: items out of stock
from (references
where appropriate).
For example:
7
Appendices
5 A short sequential Please append a copy
list of steps that you
of the audit tool (if
Methodology took to carry out 6 appropriate) used
the audit. You should
within the audit.
state your chosen
population for this
study. e.g. patients References
aged 80 years and
above List any references
that you have used
30 PS Magazine | DSQS – Declaring Compliance Buy direct online at PSUK.co.uk
09/10/2024 14:34:45
P4304.1-V103_PS Magazine - November 244.indd 30 09/10/2024 14:34:45
P4304.1-V103_PS Magazine - November 244.indd 30