Page 8 - P4304.1-V113 PS Magazine September 2025 (Print Ready)_Handy
P. 8
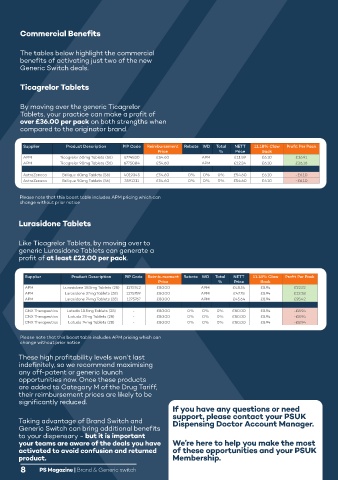
Commercial Benefits
The tables below highlight the commercial
benefits of activating just two of the new
Generic Switch deals.
Ticagrelor Tablets
PSUK is pleased to offer a 40% discount
By moving over the generic Ticagrelor on Sereflo pMDIs for dispensing practices
Tablets, your practice can make a profit of
over £36.00 per pack on both strengths when
compared to the originator brand.
Supplier Product Description PIP Code Reimbursement Rebate WD Total NETT 11.18% Claw Profit Per Pack Product NHS List price 1 PSUK discount Saving
Price % Price Back
APM Ticagrelor 60mg Tablets (56) 6774830 £54.60 APM £11.59 £6.10 £36.91 Sereflo pMDI
APM Ticagrelor 90mg Tablets (56) 6775084 £54.60 APM £12.34 £6.10 £36.16 £14.99 40% £5.99
25/125mcg
AstraZeneca Brilique 60mg Tablets (56) 4019345 £54.60 0% 0% 0% £54.60 £6.10 -£6.10 Sereflo pMDI
AstraZeneca Brilique 90mg Tablets (56) 3591211 £54.60 0% 0% 0% £54.60 £6.10 -£6.10 £19.99 40% £7.99
25/250mcg
Please note that this boost table includes APM pricing which can Sereflo pMDI can offer a 25mcg salmeterol with 125 or 250mcg fluticasone
change without prior notice combination more cost-effectively than the market-leading competitors,
Seretide® and Sirdupla® (salmeterol/fluticasone). Plus, you can take
1-3
advantage of significant additional savings with the PSUK discount.
Lurasidone Tablets
Like Ticagrelor Tablets, by moving over to Sereflo pMDI is indicated for use in adults with asthma 18 years of age and older only. 4,5
generic Lurasidone Tablets can generate a Sereflo pMDI is indicated in the regular treatment of patients with moderate to severe asthma
profit of at least £22.00 per pack. where use of a combination product (long-acting β2 agonist and inhaled corticosteroid)
is appropriate:
- patients not adequately controlled on a lower strength corticosteroid combination product 4,5
or
Supplier Product Description PIP Code Reimbursement Rebate WD Total NETT 11.18% Claw Profit Per Pack - patients already adequately controlled on an inhaled corticosteroid in a mid or
Price % Price Back
APM Lurasidone 18.5mg Tablets (28) 1275742 £80.00 APM £48.54 £8.94 £22.52 high strength and a long-acting β2 agonist. 4,5
APM Lurasidone 37mg Tablets (28) 1275759 £80.00 APM £47.78 £8.94 £23.28 Seretide is a trademark of GSK. Sirdupla is a registered trademark of Mylan.
APM Lurasidone 74mg Tablets (28) 1275767 £80.00 APM £45.64 £8.94 £25.42 pMDI = pressurised metered dose inhaler.
CNX Therapeutics Latuda 18.5mg Tablets (28) - £80.00 0% 0% 0% £80.00 £8.94 -£8.94
®
CNX Therapeutics Latuda 37mg Tablets (28) - £80.00 0% 0% 0% £80.00 £8.94 -£8.94 Sereflo Metered Dose Inhaler (salmeterol xinafoate/fluticasone with caution in patients with severe cardiovascular disorders or heart rhythm abnormalities and in
CNX Therapeutics Latuda 74mg Tablets (28) - £80.00 0% 0% 0% £80.00 £8.94 -£8.94 propionate) patients with diabetes mellitus, thyrotoxicosis, uncorrected hypokalaemia or with a predisposition to
low levels of serum potassium. Prescribers should also be aware of the risk of adrenal suppression
Prescribing Information and acute adrenal crisis which may occur in patients on prolonged treatment with high doses of
Please consult the full Summary of Product Characteristics (SmPC) before prescribing
Sereflo 25 microgram (μg) salmeterol xinafoate /125 μg or 250 μg fluticasone propionate per actuation inhaled corticosteroids. Systemic effects may occur with any inhaled corticosteroid and it is important
therefore, that the patient is reviewed regularly and the dose of inhaled corticosteroid is reduced to the
Please note that this boost table includes APM pricing which can pressurised inhalation, suspension. For both dose strengths the equivalent delivered dose per lowest dose at which effective control of asthma is maintained. Visual disturbances may be reported
change without prior notice actuation is 21 μg of salmeterol and the delivered fluticasone propionate is 110 μg, for 125 μg dose with steroid use. Patients presenting with blurred vision or other visual disturbances should be
strength and 220 μg, for 250 μg dose strength. INDICATIONS: For use in adults with asthma 18 years
of age and older only. Sereflo is indicated in the regular treatment of patients with moderate to severe considered for referral to an ophthalmologist. Drug Interactions: Concomitant use should be avoided
asthma where use of a combination product (long-acting β2 agonist and inhaled corticosteroid) is with; non-selective and selective β blockers, ritonavir and other potent/moderate CYP3A inhibitors,
These high profitability levels won’t last appropriate: patients not adequately controlled on a lower strength corticosteroid combination product unless potential benefit outweighs the risk. Particular caution is advised in acute severe asthma
as hypokalaemia may be potentiated by concomitant treatment with xanthine derivatives, steroids
or patients already adequately controlled on an inhaled corticosteroid in a mid or high strength and
indefinitely, so we recommend maximising a long-acting β2 agonist. POSOLOGY AND ADMINISTRATION: Patients should be instructed and diuretics. See the SmPC for further information on contraindications and precautions.
PREGNANCY AND LACTATION: Balance risks against benefits. UNDESIRABLE EFFECTS:
in the proper use of their inhaler (see SmPC and patient information leaflet). Recommended
any off-patent or generic launch doses in adults 18 years and older - Two inhalations of 25μg salmeterol and 125 μg or 250 μg Adverse events which have been associated with salmeterol/fluticasone propionate include: Very
common; nasopharyngitis and headache; Common; candidiasis of the mouth and throat, pneumonia,
fluticasone propionate twice daily. A short-term trial of salmeterol and fluticasone propionate may be
opportunities now. Once these products considered as initial maintenance therapy in adults with moderate persistent asthma for whom rapid bronchitis, hypokalaemia, throat irritation, hoarseness/dysphonia, sinusitis, contusions, muscle
cramps, traumatic fractures, arthralgia and myalgia. For other adverse events please consult the full
control of asthma is essential. In these cases, the recommended initial dose is two inhalations of 25
are added to Category M of the Drug Tariff, μg salmeterol and 50 μg fluticasone propionate twice daily. Note: Sereflo is not available in a lower SmPC. MARKETING AUTHORISATION HOLDER & PL NUMBERS: Cipla (EU) Ltd. Dixcart House,
Addlestone Road, Bourne Business Park, Addlestone, Surrey KT15 2LE. PL 36390/0237 and PL
strength product containing salmeterol 25 μg and fluticasone propionate 50 μg. Therefore, when
their reimbursement prices are likely to be initiating therapy, or when it is appropriate to titrate down to a dose below 125 μg, an alternative 36390/0238. Legal category: POM. NHS Cost 25 μg/125 μg 1x 120 dose MDI £14.99 and 25 μg/250
fixed-dose combination of salmeterol and fluticasone propionate containing a lower dose of the
μg 1x 120 doses MDI £19.99. Date of last revision: April 2020.
significantly reduced. inhaled corticosteroid is required. Use of a spacer device; where required in those with difficulties in
coordinating actuation of the inhaler with inspiration of breath, it is recommended ONLY for higher
If you have any questions or need strength Sereflo containing salmeterol 25 μg and fluticasone propionate 250 μg. Patients should Adverse events should be reported. Reporting forms and information can
continue to use the same make of spacer device (Volumatic or the AeroChamber Plus) as switching
support, please contact your PSUK between spacer devices can result in changes in the dose delivered to the lungs. See the SmPC be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellowcard
in the Google Play or Apple App Store. Adverse events should also be
Taking advantage of Brand Switch and Dispensing Doctor Account Manager. for further information on initiation, titration down and spacer use. CONTRAINDICATIONS: reported Cipla (EU) Ltd on 0800 0472144, drugsafety@cipla.com
Hypersensitivity to the active substances or to any of the excipients. SPECIAL WARNINGS AND
Generic Switch can bring additional benefits PRECAUTIONS: Sereflo should not be used to treat acute asthma symptoms for which a fast- and
short-acting bronchodilator is required. Patients should be advised to have their inhaler to be used
to your dispensary - but it is important for relief in an acute asthma attack available at all times. Patients should not be initiated on Sereflo References.
during an exacerbation, or significantly worsening or acutely deteriorating asthma. Serious asthma-
1. Dictionary of medicines and devices (dm+d), available at https://www.nhsbsa.nhs.uk/pharmacies-gp-
your teams are aware of the deals you have We’re here to help you make the most related adverse events and exacerbations may occur with Sereflo. Patients should be asked to practices-and-appliance-contractors/dictionary-medicines-and-devices-dmd (accessed August 2022).
2. British National Formulary, Medicinal forms, available at
continue treatment but to seek medical advice if asthma symptoms remain uncontrolled or worsen
activated to avoid confusion and returned of these opportunities and your PSUK after initiation. Treatment should not be stopped abruptly due to risk of exacerbation. Therapy should https://bnf.nice.org.uk/medicinal-forms/fluticasone-with-salmeterol.html (accessed August 2022).
3. MIMS.co.uk (accessed August 2022).
be down-titrated under physician supervision. All inhaled medication containing corticosteroids
product. Membership. should be administered with caution in patients with active or quiescent pulmonary tuberculosis and 4. Sereflo 25 microgram/125 microgram. Summary of Product Characteristics.
5. Sereflo 25 microgram/250 microgram. Summary of Product Characteristics.
fungal, viral or other infections of the airway. Salmeterol and fluticasone propionate should be used
8 PS Magazine | Brand & Generic switch August 2022 CIPSER20220021
27/08/2025 16:40:38
P4304.1-V113 PS Magazine September 2025.indd 8 27/08/2025 16:40:38
P4304.1-V113 PS Magazine September 2025.indd 8