Page 13 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 13
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
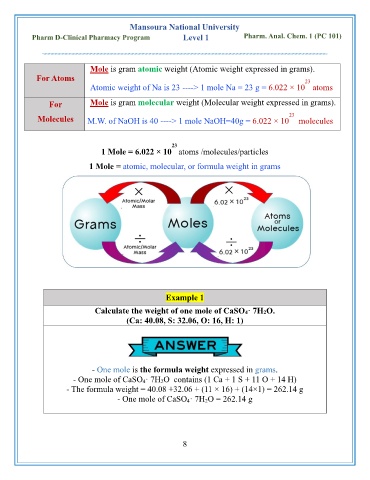
Mole is gram atomic weight (Atomic weight expressed in grams).
For Atoms 23
Atomic weight of Na is 23 ----> 1 mole Na = 23 g = 6.022 × 10 atoms
For Mole is gram molecular weight (Molecular weight expressed in grams).
23
Molecules M.W. of NaOH is 40 ----> 1 mole NaOH=40g = 6.022 × 10 molecules
23
1 Mole = 6.022 × 10 atoms /molecules/particles
1 Mole = atomic, molecular, or formula weight in grams
Example 1
Calculate the weight of one mole of CaSO4· 7H2O.
(Ca: 40.08, S: 32.06, O: 16, H: 1)
- One mole is the formula weight expressed in grams.
- One mole of CaSO 4· 7H 2O contains (1 Ca + 1 S + 11 O + 14 H)
- The formula weight = 40.08 +32.06 + (11 × 16) + (14×1) = 262.14 g
- One mole of CaSO 4· 7H 2O = 262.14 g
8