Page 32 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 32
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
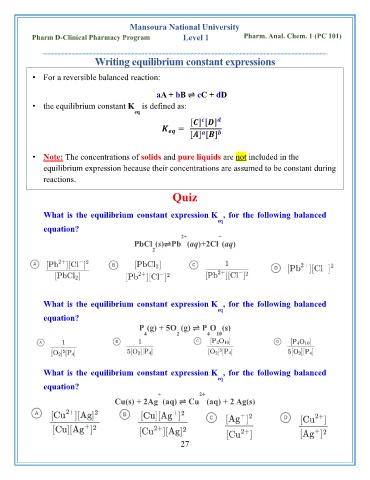
Writing equilibrium constant expressions
• For a reversible balanced reaction:
aA + bB ⇌ cC + dD
• the equilibrium constant K is defined as:
eq
[ ] [ ]
=
[ ] [ ]
• Note: The concentrations of solids and pure liquids are not included in the
equilibrium expression because their concentrations are assumed to be constant during
reactions.
Quiz
What is the equilibrium constant expression K , for the following balanced
eq
equation?
2+ −
PbCl (s)⇌Pb (aq)+2Cl (aq)
2
What is the equilibrium constant expression K , for the following balanced
eq
equation?
P (g) + 5O (g) ⇌ P O (s)
4 2 4 10
What is the equilibrium constant expression K , for the following balanced
eq
equation?
+ 2+
Cu(s) + 2Ag (aq) ⇌ Cu (aq) + 2 Ag(s)
27