Page 27 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 27
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
Reaction rate
▪ The change in concentration of a reactant or product per unit time.
Definition
▪ Some reactions take place slowly
Ex: (conversion of iron to iron oxide, Iron rusting)
▪ While others proceed very rapidly.
Notes -
Ex: (AgNO + Cl AgCl)
3
Reactions involving a combination of ions occur very rapidly, but
covalently bonded molecules or groups often react slowly.
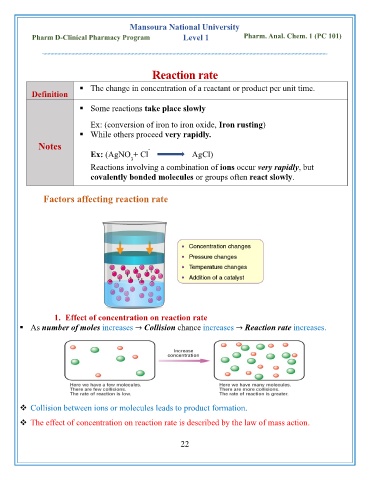
Factors affecting reaction rate
1. Effect of concentration on reaction rate
▪ As number of moles increases → Collision chance increases → Reaction rate increases.
❖ Collision between ions or molecules leads to product formation.
❖ The effect of concentration on reaction rate is described by the law of mass action.
22