Page 24 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 24
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
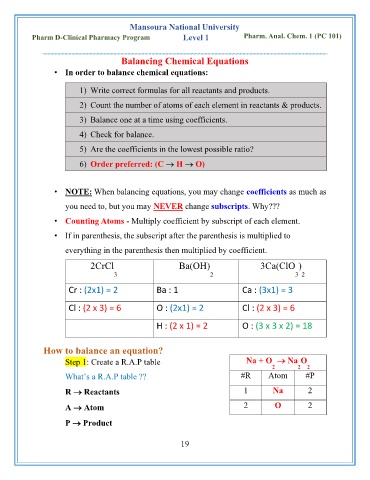
Balancing Chemical Equations
• In order to balance chemical equations:
1) Write correct formulas for all reactants and products.
2) Count the number of atoms of each element in reactants & products.
3) Balance one at a time using coefficients.
4) Check for balance.
5) Are the coefficients in the lowest possible ratio?
6) Order preferred: (C → H → O)
• NOTE: When balancing equations, you may change coefficients as much as
you need to, but you may NEVER change subscripts. Why???
• Counting Atoms - Multiply coefficient by subscript of each element.
• If in parenthesis, the subscript after the parenthesis is multiplied to
everything in the parenthesis then multiplied by coefficient.
2CrCl Ba(OH) 3Ca(ClO )
3 2 3 2
Cr : (2x1) = 2 Ba : 1 Ca : (3x1) = 3
Cl : (2 x 3) = 6 O : (2x1) = 2 Cl : (2 x 3) = 6
H : (2 x 1) = 2 O : (3 x 3 x 2) = 18
How to balance an equation?
Step 1: Create a R.A.P table Na + O → Na O
2 2 2
What’s a R.A.P table ?? #R Atom #P
R → Reactants 1 Na 2
A → Atom 2 O 2
P → Product
19