Page 20 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 20
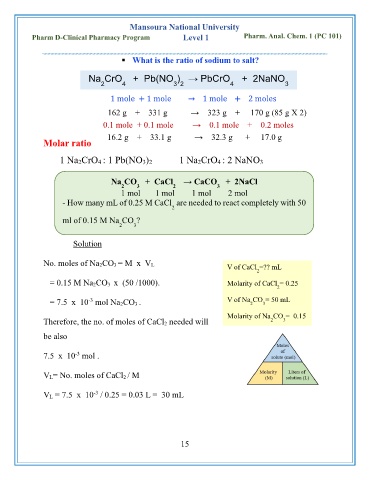
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
▪ What is the ratio of sodium to salt?
Na CrO + Pb(NO ) → PbCrO + 2NaNO
2 4 3 2 4 3
1 mole + 1 mole → 1 mole + 2 moles
162 g + 331 g → 323 g + 170 g (85 g X 2)
0.1 mole + 0.1 mole → 0.1 mole + 0.2 moles
16.2 g + 33.1 g → 32.3 g + 17.0 g
Molar ratio
1 Na2CrO4 : 1 Pb(NO3)2 1 Na2CrO4 : 2 NaNO3
Na CO + CaCl → CaCO + 2NaCl
2 3 2 3
1 mol 1 mol 1 mol 2 mol
- How many mL of 0.25 M CaCl are needed to react completely with 50
2
ml of 0.15 M Na CO ?
2 3
Solution
No. moles of Na 2CO 3 = M x V L
V of CaCl =?? mL
2
= 0.15 M Na 2CO 3 x (50 /1000). Molarity of CaCl = 0.25
2
-3
= 7.5 x 10 mol Na 2CO 3 . V of Na CO = 50 mL
2
3
Molarity of Na CO = 0.15
Therefore, the no. of moles of CaCl 2 needed will 2 3
be also
-3
7.5 x 10 mol .
V L= No. moles of CaCl 2 / M
V L = 7.5 x 10 / 0.25 = 0.03 L = 30 mL
-3
15