Page 35 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 35
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
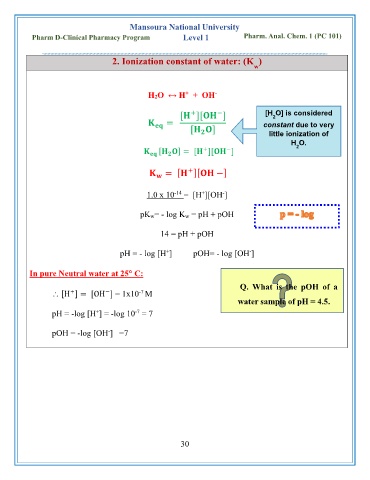
2. Ionization constant of water: (K )
w
-
+
H2O ↔ H + OH
+
−
[ ][ ] [H O] is considered
2
= constant due to very
[ ] little ionization of
H O.
2
−
+
[ ] = [ ][ ]
(water is very
+
= [ ][ −] weak
electrolyte)
+
-14
-
1.0 x 10 = [H ][OH ]
pK w= - log K w = pH + pOH
14 = pH + pOH
-
+
pH = - log [H ] pOH= - log [OH ]
In pure Neutral water at 25 C:
Q. What is the pOH of a
+
-7
−
[H ] = [OH ] = 1x10 M
water sample of pH = 4.5.
pH = -log [H ] = -log 10 = 7
+
-7
-
pOH = -log [OH ] =7
30