Page 84 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 84
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
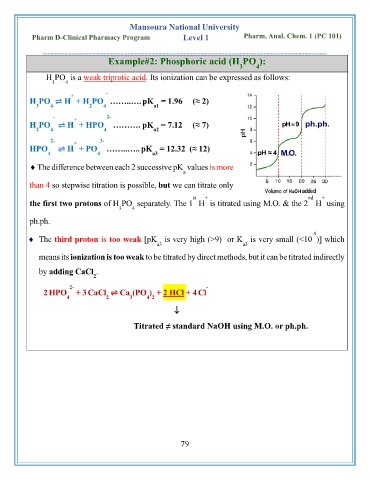
Example#2: Phosphoric acid (H PO ):
3 4
H PO is a weak triprotic acid. Its ionization can be expressed as follows:
3 4
+ -
H PO ⇌ H + H PO ……..….pK = 1.96 (≈ 2)
3 4 2 4 a1
- + 2-
H PO ⇌ H + HPO ………. pK = 7.12 (≈ 7)
2 4 4 a2
2- + 3-
HPO ⇌ H + PO ……..…..pK = 12.32 (≈ 12)
4 4 a3
The difference between each 2 successive pK values is more
a
than 4 so stepwise titration is possible, but we can titrate only
st + nd +
the first two protons of H PO separately. The 1 H is titrated using M.O. & the 2 H using
3 4
ph.ph.
-9
The third proton is too weak [pK is very high (>9) or K is very small (<10 )] which
a3 a3
means its ionization is too weak to be titrated by direct methods, but it can be titrated indirectly
by adding CaCl .
2
2- -
2HPO + 3CaCl ⇌ Ca (PO ) + 2 HCl + 4Cl
4 2 3 4 2
Titrated ≠ standard NaOH using M.O. or ph.ph.
79