Page 89 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 89
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
Summary
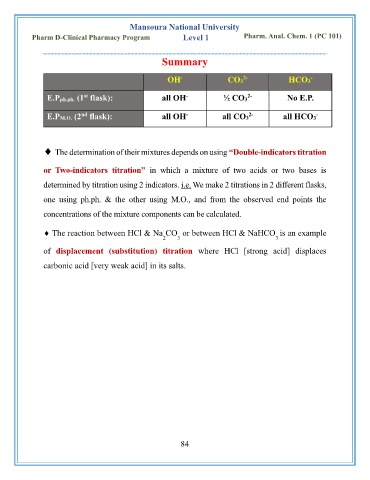
OH CO3 HCO3
-
2-
-
E.Pph.ph. (1 flask): all OH ½ CO3 No E.P.
2-
st
-
2-
E.PM.O. (2 flask): all OH all CO3 all HCO3
nd
-
-
The determination of their mixtures depends on using “Double-indicators titration
or Two-indicators titration” in which a mixture of two acids or two bases is
determined by titration using 2 indicators. i.e. We make 2 titrations in 2 different flasks,
one using ph.ph. & the other using M.O., and from the observed end points the
concentrations of the mixture components can be calculated.
The reaction between HCl & Na CO or between HCl & NaHCO is an example
2 3 3
of displacement (substitution) titration where HCl [strong acid] displaces
carbonic acid [very weak acid] in its salts.
84