Page 90 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 90
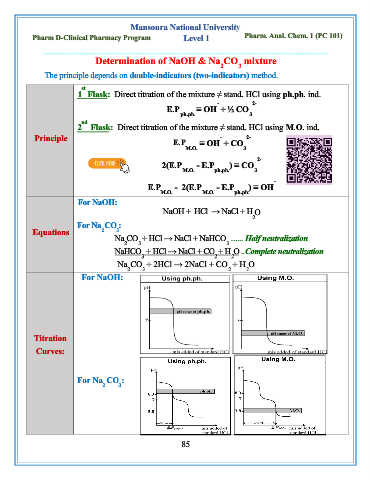
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
Determination of NaOH & Na CO mixture
2 3
The principle depends on double-indicators (two-indicators) method.
st
1 Flask: Direct titration of the mixture ≠ stand. HCl using ph.ph. ind.
- 2-
E.P ≡ OH + ½ CO
ph.ph. 3
nd
2 Flask: Direct titration of the mixture ≠ stand. HCl using M.O. ind.
Principle - 2-
E.P ≡ OH + CO
M.O. 3
2-
2(E.P - E.P ) ≡ CO
M.O. ph.ph. 3
-
E.P - 2(E.P - E.P ) ≡ OH
M.O. M.O. ph.ph.
For NaOH:
NaOH + HCl → NaCl + H O
2
For Na CO :
Equations 2 3
Na CO + HCl → NaCl + NaHCO …... Half neutralization
2 3 3
NaHCO + HCl → NaCl + CO + H O ..Complete neutralization
3 2 2
Na CO + 2HCl → 2NaCl + CO + H O
2 3 2 2
For NaOH:
Titration
Curves:
For Na CO :
2 3
85