Page 86 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 86
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
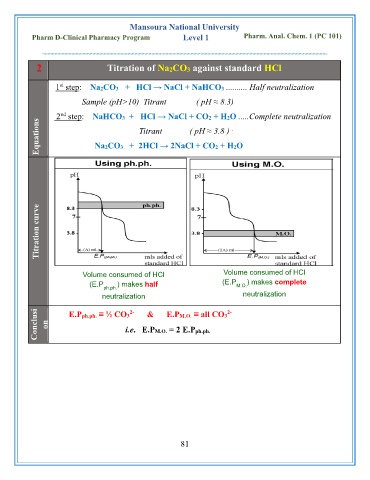
2 Titration of Na2CO3 against standard HCl
st
1 step: Na2CO3 + HCl → NaCl + NaHCO3 .......... Half neutralization
Sample (pH>10) Titrant ( pH ≈ 8.3)
2 step: NaHCO3 + HCl → NaCl + CO2 + H2O .....Complete neutralization
nd
Equations Titrant ( pH ≈ 3.8 )
.
Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
Titration curve
Volume consumed of HCl Volume consumed of HCl
(E.P ) makes complete
(E.P ph.ph. ) makes half M.O.
neutralization neutralization
Conclusi on E.Pph.ph. ≡ ½ CO3 & E.PM.O. ≡ all CO3
2-
2-
i.e. E.PM.O. = 2 E.Pph.ph.
81