Page 7 - Interactive Theoritical Notes of Bioharmaceutics and pharamcokinetics.docx compressed
P. 7
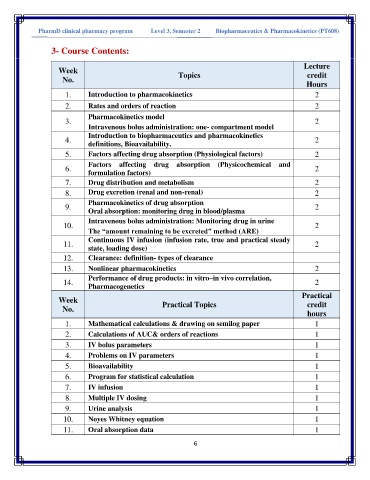
PharmD clinical pharmacy program Level 3, Semester 2 Biopharmaceutics & Pharmacokinetics (PT608(
3- Course Contents:
Lecture
Week
No. Topics credit
Hours
1. Introduction to pharmacokinetics 2
2. Rates and orders of reaction 2
Pharmacokinetics model
3. 2
Intravenous bolus administration: one- compartment model
Introduction to biopharmaceutics and pharmacokinetics
4. 2
definitions, Bioavailability.
5. Factors affecting drug absorption (Physiological factors) 2
Factors affecting drug absorption (Physicochemical and
6. formulation factors) 2
7. Drug distribution and metabolism 2
8. Drug excretion (renal and non-renal) 2
Pharmacokinetics of drug absorption
9. 2
Oral absorption: monitoring drug in blood/plasma
Intravenous bolus administration: Monitoring drug in urine
10. 2
The “amount remaining to be excreted” method (ARE)
Continuous IV infusion (infusion rate, true and practical steady
11. 2
state, loading dose)
12. Clearance: definition- types of clearance
13. Nonlinear pharmacokinetics 2
Performance of drug products: in vitro–in vivo correlation,
14. 2
Pharmacogenetics
Practical
Week Practical Topics credit
No.
hours
1. Mathematical calculations & drawing on semilog paper 1
2. Calculations of AUC& orders of reactions 1
3. IV bolus parameters 1
4. Problems on IV parameters 1
5. Bioavailability 1
6. Program for statistical calculation 1
7. IV infusion 1
8. Multiple IV dosing 1
9. Urine analysis 1
10. Noyes Whitney equation 1
11. Oral absorption data 1
6