Page 102 - DUOKOPT BIBLIOBOOK
P. 102
DUOKOPT - bibliography book - CONFIDENTIAL - document for exclusive use by personnel of Laboratoires Théa – DO NOT DISTRIBUTE
®
blockers have served as standard ocular hypotensive agents
for many years, these medicines can provoke cardiovascu-
lar, pulmonary, central nervous system, and endocrine side
effects. Dorzolamide is a safe, effective, and easily toler-
7
ated topical carbonic anhydrase inhibitor that retains the
ocular hypotensive actions of systemic carbonic anhydrase
inhibition without the accompanying side effects. 2,3 In
comparative trials, dorzolamide is equal to blockade for
reduction of intraocular pressure. 2,3
Because single-drug treatment may not sufficiently re-
duce intraocular pressure for some patients with primary
open-angle glaucoma, two unrelated agents may be com-
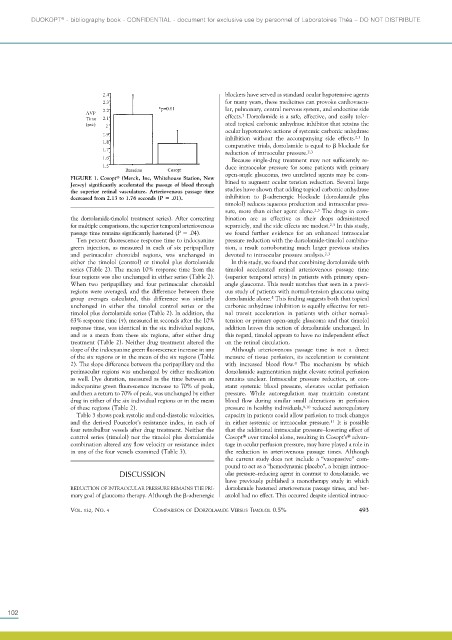
FIGURE 1. Cosopt (Merck, Inc, Whitehouse Station, New bined to augment ocular tension reduction. Several large
Jersey) significantly accelerated the passage of blood through
the superior retinal vasculature. Arteriovenous passage time studies have shown that adding topical carbonic anhydrase
decreased from 2.13 to 1.76 seconds (P .01). inhibition to -adrenergic blockade (dorzolamide plus
timolol) reduces aqueous production and intraocular pres-
sure, more than either agent alone. 2,3 The drugs in com-
the dorzolamide-timolol treatment series). After correcting bination are as effective as their drops administered
for multiple comparisons, the superior temporal arteriovenous separately, and the side effects are modest. 2,3 In this study,
passage time remains significantly hastened (P .04). we found further evidence for an enhanced intraocular
Ten percent fluorescence response time to indocyanine pressure reduction with the dorzolamide-timolol combina-
green injection, as measured in each of six peripapillary tion, a result corroborating much larger previous studies
and perimacular choroidal regions, was unchanged in devoted to intraocular pressure analysis. 2,3
either the timolol (control) or timolol plus dorzolamide In this study, we found that combining dorzolamide with
series (Table 2). The mean 10% response time from the timolol accelerated retinal arteriovenous passage time
four regions was also unchanged in either series (Table 2). (superior temporal artery) in patients with primary open-
When two peripapillary and four perimacular choroidal angle glaucoma. This result matches that seen in a previ-
regions were averaged, and the difference between these ous study of patients with normal-tension glaucoma using
group averages calculated, this difference was similarly dorzolamide alone. This finding suggests both that topical
5
unchanged in either the timolol control series or the carbonic anhydrase inhibition is equally effective for reti-
timolol plus dorzolamide series (Table 2). In addition, the nal transit acceleration in patients with either normal-
63% response time ( ), measured in seconds after the 10% tension or primary open-angle glaucoma and that timolol
response time, was identical in the six individual regions, addition leaves this action of dorzolamide unchanged. In
and as a mean from these six regions, after either drug this regard, timolol appears to have no independent effect
treatment (Table 2). Neither drug treatment altered the on the retinal circulation.
slope of the indocyanine green fluorescence increase in any Although arteriovenous passage time is not a direct
of the six regions or in the mean of the six regions (Table measure of tissue perfusion, its acceleration is consistent
2). The slope difference between the peripapillary and the with increased blood flow. The mechanism by which
8
perimacular regions was unchanged by either medication dorzolamide augmentation might elevate retinal perfusion
as well. Dye duration, measured as the time between an remains unclear. Intraocular pressure reduction, at con-
indocyanine green fluorescence increase to 70% of peak, stant systemic blood pressure, elevates ocular perfusion
and then a return to 70% of peak, was unchanged by either pressure. While autoregulation may maintain constant
drug in either of the six individual regions or in the mean blood flow during similar small alterations in perfusion
of these regions (Table 2). pressure in healthy individuals, 9,10 reduced autoregulatory
Table 3 shows peak systolic and end-diastolic velocities, capacity in patients could allow perfusion to track changes
and the derived Pourcelot’s resistance index, in each of in either systemic or intraocular pressure. 11 It is possible
four retrobulbar vessels after drug treatment. Neither the that the additional intraocular pressure–lowering effect of
control series (timolol) nor the timolol plus dorzolamide Cosopt over timolol alone, resulting in Cosopt’s advan-
combination altered any flow velocity or resistance index tage in ocular perfusion pressure, may have played a role in
in any of the four vessels examined (Table 3). the reduction in arteriovenous passage times. Although
the current study does not include a “vasopassive” com-
pound to act as a “hemodynamic placebo”, a benign intraoc-
DISCUSSION ular pressure–reducing agent in contrast to dorzolamide, we
have previously published a monotherapy study in which
REDUCTION OF INTRAOCULAR PRESSURE REMAINS THE PRI- dorzolamide hastened arteriovenous passage times, and bet-
mary goal of glaucoma therapy. Although the -adrenergic axolol had no effect. This occurred despite identical intraoc-
VOL. 132,NO. 4 COMPARISON OF DORZOLAMIDE VERSUS TIMOLOL 0.5% 493
102