Page 3 - MSC & Exosomes in autoimmune
P. 3
3556 Chung et al.
Angiography
A 5F MPA catheter (Cook) was introduced over a
0.035-in. guidewire (UniQual 150-cm angled; Asahi Intecc,
Aichi, Japan) through the sheath and advanced into the verte-
bral artery. A multipurpose angiographic (MPA) catheter was
left in place at the distal portion of the vertebral artery, and a
2.2F microcatheter (Stride microcatheter, 2.2F, 150 cm;
Asahi) was introduced over a 0.014-in. microguidewire (Stride
microguidewire, 0.014-in., 180 cm; Asahi) through the 5F
MPA catheter into the proximal basilar artery. Contrast
medium (Ominpaque; GE Healthcare, Waukesha, WI) was
injected to confirm the intact condition of the cerebral arteries
prior to the occlusion procedure and to confirm the occlusion
of the middle cerebral artery (MCA) 30 min after the occlu-
sion procedure.
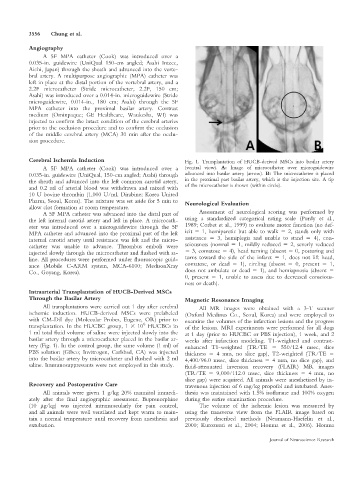
Cerebral Ischemia Induction Fig. 1. Transplantation of HUCB-derived MSCs into basilar artery
A 5F MPA catheter (Cook) was introduced over a (ventral view). A: Image of microcatheter over microguidewire
0.035-in. guidewire (UniQual, 150-cm angled; Asahi) through advanced into basilar artery (arrow). B: The microcatheter is placed
the sheath and advanced into the left common carotid artery, in the proximal part basilar artery, which is the injection site. A tip
and 0.2 ml of arterial blood was withdrawn and mixed with of the microcatheter is shown (within circle).
10 U bovine thrombin (1,000 U/ml, Dirabine; Korea United
Pharm, Seoul, Korea). The mixture was set aside for 5 min to Neurological Evaluation
allow clot formation at room temperature.
A 5F MPA catheter was advanced into the distal part of Assessment of neurological scoring was performed by
the left internal carotid artery and left in place. A microcath- using a standardized categorical rating scale (Purdy et al.,
eter was introduced over a microguidewire through the 5F 1989; Corbet et al., 1999) to evaluate motor function (no def-
MPA catheter and advanced into the proximal part of the left icit 5 1, hemiparetic but able to walk 5 2, stands only with
internal carotid artery until resistance was felt and the micro- assistance 5 3, hemiplegia and unable to stand 5 4), con-
catheter was unable to advance. Thrombus emboli were sciousness (normal 5 1, mildly reduced 5 2, severly reduced
injected slowly through the microcatheter and flushed with sa- 5 3, comatose 5 4), head turning (absent 5 0, posturing and
line. All procedures were performed under fluoroscopic guid- turns toward the side of the infarct 5 1, does not lift head,
ance (Mobile C-ARM system, MCA-6100; MedisonXray comatose, or dead 5 1), circling (absent 5 0, present 5 1,
Co., Goyang, Korea). does not ambulate or dead 5 1), and hemiapnosia (absent 5
0, present 5 1, unable to assess due to decreased conscious-
ness or death).
Intraarterial Transplantation of HUCB-Derived MSCs
Through the Basilar Artery Magnetic Resonance Imaging
All transplantations were carried out 1 day after cerebral All MR images were obtained with a 3-T scanner
ischemic induction. HUCB-derived MSCs were prelabeled (Oxford Medinus Co., Seoul, Korea) and were employed to
with CM-DiI dye (Molecular Probes, Eugene, OR) prior to examine the volumes of the infarction lesions and the progress
6
transplantation. In the HUCBC group, 1 3 10 HUCBCs in of the lesions. MRI experiments were performed for all dogs
1 ml total fluid volume of saline were injected slowly into the at 1 day (prior to HUCBC or PBS injection), 1 week, and 2
basilar artery through a microcatheter placed in the basilar ar- weeks after infarction modeling. T1-weighted and contrast-
tery (Fig. 1). In the control group, the same volume (1 ml) of enhanced T1-weighted (TR/TE 5 550/12.4 msec, slice
PBS solution (Gibco; Invitrogen, Carlsbad, CA) was injected thickness 5 4 mm, no slice gap), T2-weighted (TR/TE 5
into the basilar artery by microcatheter and flushed with 2 ml 4,400/96.0 msec, slice thickness 5 4 mm, no slice gap), and
saline. Immunosuppressants were not employed in this study. fluid-attenuated inversion recovery (FLAIR) MR images
(TR/TE 5 9,000/112.0 msec, slice thickness 5 4 mm, no
slice gap) were acquired. All animals were anesthetized by in-
Recovery and Postoperative Care travenous injection of 6 mg/kg propofol and intubated. Anes-
All animals were given 1 g/kg 20% mannitol immedi- thesia was maintained with 1.5% isoflurane and 100% oxygen
ately after the final angiographic assessment. Buprenorphine during the entire examination procedure.
(10 lg/kg) was injected intramuscularly for pain control, The volume of the ischemic lesion was measured by
and all animals were well ventilated and kept warm to main- using the transverse view from the FLAIR image based on
tain a normal temperature until recovery from anesthesia and previously described methods (Neumann-Haefelin et al.,
extubation. 2000; Kurozumi et al., 2004; Honma et al., 2006). Honma
Journal of Neuroscience Research