Page 3 - The Hemophilia Times 2.0_Neat
P. 3
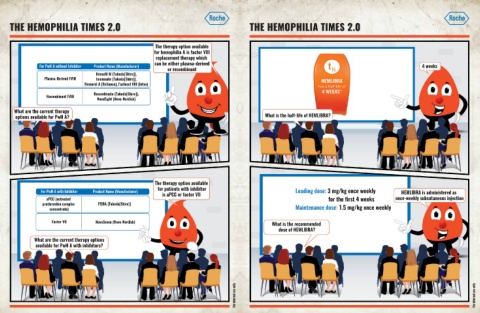
The therapy option available
for hemophilia A is factor VIII
replacement therapy which
can be either plasma-derived FVIII product type Product name (manufacturer)
For PwH A without Inhibitor Product Name (Manufacturer) 4 weeks
or recombinant
Hemofil M (Takeda[Shire]),
Plasma-Derived FVIII Immunate (Takeda[Shire]), HEMLIBRA
Hemorel A (Reliance), Factocel VIII (Intas) has a half-life of
Recombinant 10
Recombinate (Takeda[Shire]), FVIII 4 WEEKS
Recombinant FVIII
NovoEight (Novo-Nordisk)
What are the current therapy
options available for PwH A? What is the half-life of HEMLIBRA?
The therapy option available
for patients with inhibitor
For PwH A with Inhibitor Product Name (Manufacturer) Loading dose: 3 mg/kg once weekly HEMLIBRA is administered as
is aPCC or factor VII
aPCC (activated for the first 4 weeks once-weekly subcutaneous injection
prothrombin complex FEIBA (Takeda[Shire])
concentrate) Maintenance dose: 1.5 mg/kg once weekly
Factor VII NovoSeven (Novo-Nordisk) What is the recommended
dose of HEMLIBRA?
What are the current therapy options
available for PwH A with inhibitors?
For internal use only For internal use only