Page 4 - The Hemophilia Times 2.0_Neat
P. 4
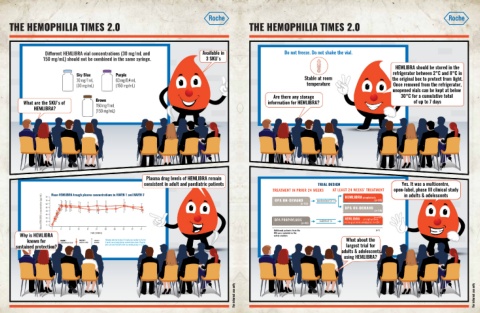
Different HEMLIBRA vial concentrations (30 mg/mL and Available in Do not freeze. Do not shake the vial.
150 mg/mL) should not be combined in the same syringe. 3 SKU's
HEMLIBRA should be stored in the
Sky Blue Purple refrigerator between 2°C and 8°C in
30 mg/1 mL 60 mg/0.4 mL Stable at room the original box to protect from light.
(30 mg/mL) (150 mg/mL) temperature Once removed from the refrigerator,
unopened vials can be kept at below
Are there any storage 30°C for a cumulative total
What are the SKU's of Brown information for HEMLIBRA? of up to 7 days
HEMLIBRA? 150 mg/1 mL
(150 mg/mL)
Plasma drug levels of HEMLIBRA remain
consistent in adult and paediatric patients TRIAL DESIGN Yes. It was a multicentre,
Mean HEMLIBRA trough plasma concentrations in HAVEN 1 and HAVEN 2 10 TREATMENT IN PRIOR 24 WEEKS AT LEAST 24 WEEKS’ TREATMENT open-label, phase III clinical study
70 70
80
in adults & adolescents
Mean HEMLIBRA trough plasma concentrations in HAVEN 1 and HAVEN 2
Mean HEMLIBRA concentration (µg/mL) 70 Mean HEMLIBRA concentration (µg/mL) 50 0 10 10 0 0 BPA PROPHYLAXIS (n=53) randomised 2:1 to Intra-patient analysis: n=24
70
80
HEMLIBRA prophylaxis
BPA ON-DEMAND
60
(n=35)
60
40
BPA ON-DEMAND
50
30
(n=18)
40
20
30
10
(n=49)
HEMLIBRA
20
prophylaxis
switched to
10
(n=49)
33
37
41
21
29
0
25
33
37
41
TIME (WEEKS)
NIS were included in the
Why is HEMLIBRA 1 5 1 9 5 HAVEN 1 13 9 17 13 HAVEN 2* 21 17 TIME (WEEKS) 29 25 Following induction dosing of 3 mg/kg once weekly for the first Additional patients from the (n=7)
mg/kg once weekly for the first
Following induction dosing of 3 mg/kg once weekly for the first
Following induction dosing of 3
HAVEN 2*
HAVEN 2*
safety analysis
VEN 1
HAVEN 1
H
A
Standard
iatric study
4 weeks, mean trough plasma concentrations above 50 µg/mL
4 weeks, mean t
p
paediatric study
aed
ough plasma concentrations above 50 µg/mL
r
t/adolescent study
l
adu
adult/adolescent study
4 weeks, mean trough plasma concentrations above 50 µg/mL
known for HAVEN 1 adult/adolescent study paediatric study deviation were sustained thereafter with once weekly dosing of 1.5 mg/kg 10 What about the
were sustained thereafter with once weekly dosing of 1.5 mg/kg 10
Following induction dosing of 3 mg/kg once weekly for the first
Standard
were sustained thereafter with once weekly dosing of 1.5 mg/kg 10
Standard HAVEN 2
Standard
4 weeks, mean trough plasma concentrations above 50 µg/mL
deviation
deviation paediatric study
deviation
adult/adolescent study
sustained protection? were sustained thereafter with once weekly dosing of 1.5 mg/kg largest trial for
adults & adolescents
using HEMLIBRA?
For internal use only For internal use only