Page 80 - Diamicron MR MIG Cycle 2(20-21) Final
P. 80
dia bet es rese ar ch a n d c li nic a l p ra ct ice 163 ( 202 0) 10 8154 9
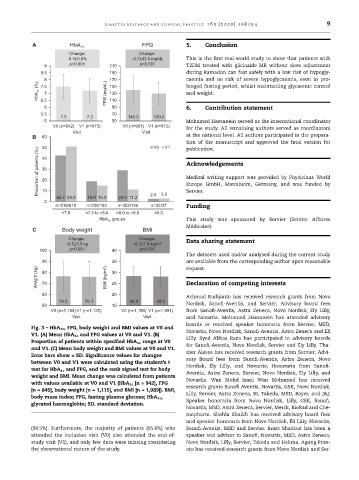
A HbA 1c FPG 5. Conclusion
Change: Change:
-0.3±0.8% -9.7±43.6 mg/dL This is the first real-world study to show that patients with
9 p<0.001 210 p<0.001 T2DM treated with gliclazide MR without dose adjustment
8.5 190 during Ramadan can fast safely with a low risk of hypogly-
8 170 caemia and no risk of severe hypoglycaemia, even in pro-
(%) 7.5 150 longed fasting period, whilst maintaining glycaemic control
HbA 1c 6.5 7 FPG (mg/dL) 130 and weight.
110
6 90 6. Contribution statement
5.5 7.5 7.2 70 140.3 130.2
5 50 Mohamed Hassanein served as the international coordinator
V0 (n=942) V1 (n=973) V0 (n=961) V1 (n=972) for the study. All remaining authors served as coordinators
Visit Visit
B 60 at the national level. All authors participated in the prepara-
tion of the manuscript and approved the final version for
50 V0 V1
) publication.
%
(
s
t 40
n
e
i Acknowledgements
t
a
p 30
f
o
n Medical writing support was provided by Physicians World
o 20
i
t
r Europe GmbH, Mannheim, Germany, and was funded by
o
p
o 10 Servier.
r 3.0
P 42.7 50.9 18.9 15.0 29.0 11.2 2.6
0
n=518/618 n=230/182 n=352/136 n=32/37 Funding
<7.5 ≥7.5 to <8.0 ≥8.0 to <9.0 ≥9.0
HbA 1c groups This study was sponsored by Servier (Servier Affaires
C Body weight BMI Me ´dicales).
Change: Change:
-0.5±3.9 kg -0.2±1.5 kg/m 2 Data sharing statement
100 p<0.001 40 p<0.001
The datasets used and/or analysed during the current study
90 35 are available from the corresponding author upon reasonable
(kg) 80 30 request.
Weight 70 BMI (kg/m 2 ) 25 Declaration of competing interests
60 20 Achmad Rudijanto has received research grants from Novo
76.5 76.1 28.4 28.2
50 15 Nordisk, Sanofi-Aventis, and Servier; Advisory board fees
V0 (n=1,189) V1 (n=1,120) V0 (n=1,159) V1 (n=1,091) from Sanofi-Aventis, Astra Zeneca, Novo Nordisk, Ely Lilly,
Visit Visit and Novartis. Mohamed Hassanein has attended advisory
boards or received speaker honoraria from Servier, MSD,
Fig. 3 – HbA 1c , FPG, body weight and BMI values at V0 and
Novartis, Novo Nordisk, Sanofi-Aventis, Astra Zeneca and Eli
V1. (A) Mean HbA 1c and FPG values at V0 and V1. (B)
Lilly. Syed Abbas Raza has participated in advisory boards
Proportion of patients within specified HbA 1c range at V0
for Sanofi-Aventis, Novo Nordisk, Servier and Ely Lilly. Tha-
and V1. (C) Mean body weight and BMI values at V0 and V1.
mer Alessa has received research grants from Servier; Advi-
Error bars show ± SD. Significance values for changes
sory Board fees from Sanofi-Aventis, Astra Zeneca, Novo
between V0 and V1 were calculated using the student’s t
test for HbA 1c and FPG, and the rank signed test for body Nordisk, Ely Lilly, and Novartis; Honoraria from Sanofi-
Aventis, Astra Zeneca, Servier, Novo Nordisk, Ely Lilly, and
weight and BMI. Mean change was calculated from patients
with values available at V0 and V1 (HbA 1c [n = 942], FPG Novartis. Wan Mohd Izani Wan Mohamed has received
research grants Sanofi Aventis, Novartis, GSK, Novo Nordisk,
[n = 845], body weight [n = 1,115], and BMI [n = 1,089]). BMI,
body mass index; FPG, fasting plasma glucose; HbA 1c , Lilly, Servier, Astra Zeneca, BI, Takeda, MSD, Bayer, and J&J;
Speaker honoraria from Novo Nordisk, Lilly, GSK, Sanofi,
glycated haemoglobin; SD, standard deviation.
Novartis, MSD, Astra Zeneca, Servier, Merck, BioRad and Che-
mopharm. Shehla Shaikh has received advisory board fees
and speaker honoraria from Novo Nordisk, Eli Lilly, Novartis,
(99.5%). Furthermore, the majority of patients (95.6%) who Sanofi-Aventis, MSD and Servier. Inass Shaltout has been a
attended the inclusion visit (V0) also attended the end-of- speaker and advisor to Sanofi, Novartis, MSD, Astra Zeneca,
study visit (V1), and only few data were missing considering Novo Nordisk, Lilly, Servier, Takeda and Hekma. Agung Pran-
the observational nature of the study. oto has received research grants from Novo Nordisk and Ser-