Page 78 - Diamicron MR MIG Cycle 2(20-21) Final
P. 78
dia bet es rese ar ch a n d c li nic a l p ra ct ice 163 ( 202 0) 10 8154 7
Table 1 – (continued)
Patients (N = 1214)
Disease history
Disease duration (years)
Mean (SD) 5.4 (5.7)
Disease duration in classes (years), n (%)
<5 years 358 (29.5)
5 and 10 years 131 (10.8)
>10 years 107 (8.8)
Missing data 618 (50.9)
IDF-DAR risk categories, n (%)
Category 1: Very high risk 47 (3.9)
Category 2: High risk 155 (12.8)
Category 3: Moderate/low risk 993 (81.8)
Missing data 19 (1.6)
Patients advised not to fast, n (%)
No 913 (75.2)
Yes 297 (24.5)
Missing data 4 (0.3)
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; IDF-DAR, International
Diabetes Federation - Diabetes and Ramadan; SD, standard deviation.
* Categories with 50 events.
point was the proportion of patients with 1 symptomatic
HE (either suggestive or confirmed), and secondary endpoints
included examination of HEs of any type as well as severe
HEs. The incidence of HEs was considered to be low in this
study, which is in alignment with other studies investigating
treatment with gliclazide (IR or MR) during Ramadan. A previ-
ous study examining hypoglycaemia in Muslims with T2DM
receiving sitagliptin or an SU during Ramadan showed that
only 6.6% of patients treated with gliclazide (IR or MR)
reported symptomatic HEs compared with 12.4% for glimepir-
ide and 19.7% for glibenclamide [7]. Another large observa-
tional study analysing the incidence of HEs in patients with
T2DM treated with glimepiride, gliclazide (IR or MR), or gliben-
clamide showed that 14.0% of those treated with gliclazide
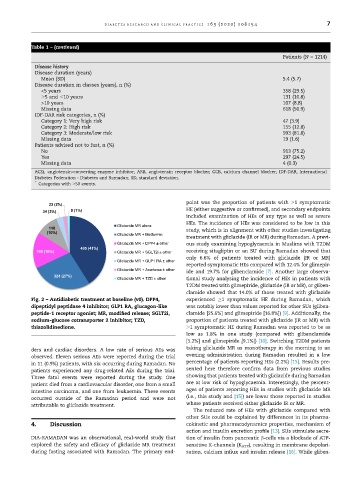
Fig. 2 – Antidiabetic treatment at baseline (v0). DPP4, experienced 1 symptomatic HE during Ramadan, which
dipeptidyl peptidase 4 inhibitor; GLP1 RA, glucagon-like was notably lower than values reported for other SUs (gliben-
peptide-1 receptor agonist; MR, modified release; SGLT2i, clamide [25.6%] and glimepiride [16.8%]) [9]. Additionally, the
sodium-glucose cotransporter 2 inhibitor; TZD, proportion of patients treated with gliclazide (IR or MR) with
thiazolidinedione. 1 symptomatic HE during Ramadan was reported to be as
low as 1.8% in one study (compared with glibenclamide
[5.2%] and glimepiride [9.1%]) [10]. Switching T2DM patients
ders and cardiac disorders. A low rate of serious AEs was taking gliclazide MR as monotherapy in the morning to an
observed. Eleven serious AEs were reported during the trial evening administration during Ramadan resulted in a low
in 11 (0.9%) patients, with six occurring during Ramadan. No percentage of patients reporting HEs (2.2%) [15]. Results pre-
patients experienced any drug-related AEs during the trial. sented here therefore confirm data from previous studies
Three fatal events were reported during the study. One showing that patients treated with gliclazide during Ramadan
patient died from a cardiovascular disorder, one from a small are at low risk of hypoglycaemia. Interestingly, the percent-
intestine carcinoma, and one from leukaemia. These events ages of patients reporting HEs in studies with gliclazide MR
(i.e., this study and [15]) are lower those reported in studies
occurred outside of the Ramadan period and were not
attributable to gliclazide treatment. where patients received either gliclazide IR or MR.
The reduced rate of HEs with gliclazide compared with
other SUs could be explained by differences in its pharma-
4. Discussion cokinetic and pharmacodynamics properties, mechanism of
action and insulin excretion profile [13]. SUs stimulate secre-
DIA-RAMADAN was an observational, real-world study that tion of insulin from pancreatic b-cells via a blockade of ATP-
explored the safety and efficacy of gliclazide MR treatment sensitive K-channels (K ATP ), resulting in membrane depolari-
during fasting associated with Ramadan. The primary end- sation, calcium influx and insulin release [16]. While gliben-