Page 74 - Diamicron MR MIG Cycle 2(20-21) Final
P. 74
dia bet es rese ar ch a n d c li nic a l p ra ct ice 163 ( 202 0) 10 8154 3
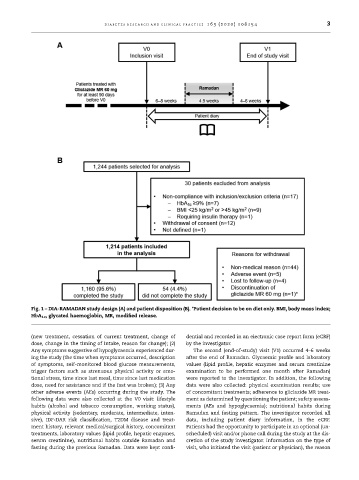
Fig. 1 – DIA-RAMADAN study design (A) and patient disposition (B). *Patient decision to be on diet only. BMI, body mass index;
HbA 1c , glycated haemoglobin, MR, modified release.
(new treatment, cessation of current treatment, change of dential and recorded in an electronic case report form (eCRF)
dose, change in the timing of intake, reason for change); (2) by the investigator.
Any symptoms suggestive of hypoglycaemia experienced dur- The second (end-of-study) visit (V1) occurred 4–6 weeks
ing the study (the time when symptoms occurred, description after the end of Ramadan. Glycaemic profile and laboratory
of symptoms, self-monitored blood glucose measurements, values (lipid profile, hepatic enzymes and serum creatinine
trigger factors such as strenuous physical activity or emo- examination to be performed one month after Ramadan)
tional stress, time since last meal, time since last medication were reported to the investigator. In addition, the following
dose, need for assistance and if the fast was broken); (3) Any data were also collected: physical examination results; use
other adverse events (AEs) occurring during the study. The of concomitant treatments; adherence to gliclazide MR treat-
following data were also collected at the V0 visit: lifestyle ment as determined by questioning the patient; safety assess-
habits (alcohol and tobacco consumption, working status), ments (AEs and hypoglycaemia); nutritional habits during
physical activity (sedentary, moderate, intermediate, inten- Ramadan and fasting pattern. The investigator recorded all
sive), IDF-DAR risk classification, T2DM disease and treat- data, including patient diary information, in the eCRF.
ment history, relevant medical/surgical history, concomitant Patients had the opportunity to participate in an optional (un-
treatments, laboratory values (lipid profile, hepatic enzymes, scheduled) visit and/or phone call during the study at the dis-
serum creatinine), nutritional habits outside Ramadan and cretion of the study investigator. Information on the type of
fasting during the previous Ramadan. Data were kept confi- visit, who initiated the visit (patient or physician), the reason