Page 42 - PC 101 practical notes 24-25..
P. 42
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
Summary
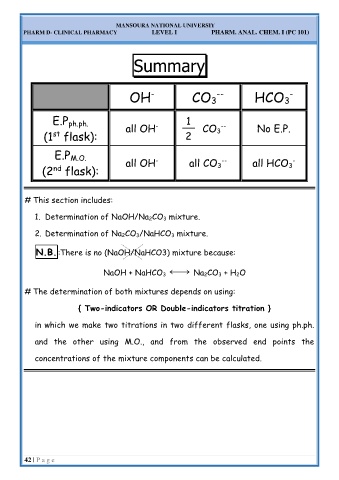
OH - CO 3 -- HCO 3 -
E.P ph.ph. - 1 --
(1 flask): all OH 2 CO No E.P.
3
st
E.P M.O. - -- -
(2 flask): all OH all CO all HCO
3
3
nd
# This section includes:
1. Determination of NaOH/Na 2CO 3 mixture.
2. Determination of Na 2CO 3/NaHCO 3 mixture.
N N. .B B. .:There is no (NaOH/NaHCO3) mixture because:
⎯→
NaOH + NaHCO 3 Na 2CO 3 + H 2O
# The determination of both mixtures depends on using:
{ Two-indicators OR Double-indicators titration }
in which we make two titrations in two different flasks, one using ph.ph.
and the other using M.O., and from the observed end points the
concentrations of the mixture components can be calculated.
42 | P a g e