Page 9 - Group 4_Project_CESP2021
P. 9
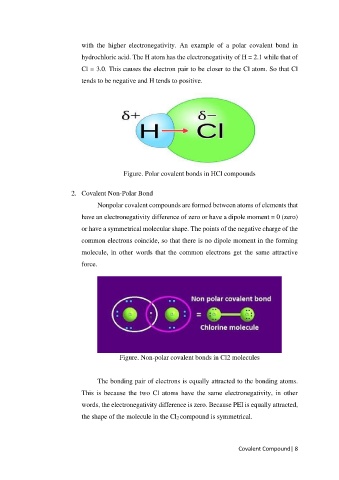
with the higher electronegativity. An example of a polar covalent bond in
hydrochloric acid. The H atom has the electronegativity of H = 2.1 while that of
Cl = 3.0. This causes the electron pair to be closer to the Cl atom. So that Cl
tends to be negative and H tends to positive.
Figure. Polar covalent bonds in HCl compounds
2. Covalent Non-Polar Bond
Nonpolar covalent compounds are formed between atoms of elements that
have an electronegativity difference of zero or have a dipole moment = 0 (zero)
or have a symmetrical molecular shape. The points of the negative charge of the
common electrons coincide, so that there is no dipole moment in the forming
molecule, in other words that the common electrons get the same attractive
force.
Figure. Non-polar covalent bonds in Cl2 molecules
The bonding pair of electrons is equally attracted to the bonding atoms.
This is because the two Cl atoms have the same electronegativity, in other
words, the electronegativity difference is zero. Because PEI is equally attracted,
the shape of the molecule in the Cl2 compound is symmetrical.
Covalent Compound| 8