Page 121 - Pengembangan E-Modul Berbasis STEAM Pada Materi Asam Basa_Nur Sirril Hayat
P. 121
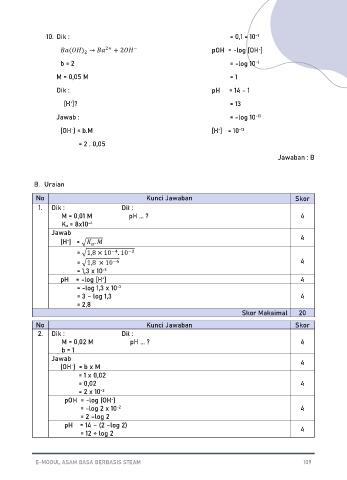
10. Dik : = 0,1 = 10
-1
-
−
( ) → 2+ + 2 pOH = -log [OH ]
2
b = 2 = -log 10 -1
M = 0,05 M = 1
Dik : pH = 14 - 1
[H ]? = 13
+
Jawab : = -log 10 -13
[OH ] = b.M [H ] = 10 -13
-
+
= 2 . 0,05
Jawaban : B
B. Uraian
No Kunci Jawaban Skor
1. Dik : Dit :
M = 0,01 M pH ... ? 4
Ka = 8x10 -4
Jawab
[H ] = √ . 4
+
= √1,8 × 10 . 10
−2
−4
= √1,8 × 10 4
−6
= 1,3 x 10
-3
pH = -log [H ] 4
+
= -log 1,3 x 10 -3
= 3 – log 1,3 4
= 2,8
Skor Maksimal 20
No Kunci Jawaban Skor
2. Dik : Dit :
M = 0,02 M pH ... ? 4
b = 1
Jawab 4
[OH ] = b x M
-
= 1 x 0,02
= 0,02 4
= 2 x 10 -2
pOH = -log [OH ]
-
= -log 2 x 10 -2 4
= 2 –log 2
pH = 14 – (2 –log 2)
= 12 + log 2 4
E-MODUL ASAM BASA BERBASIS STEAM 109