Page 8 - E-LKM ASAM BASA
P. 8
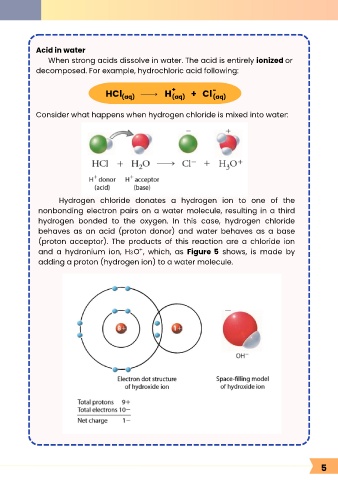
Acid in water
When strong acids dissolve in water. The acid is entirely ionized or
decomposed. For example, hydrochloric acid following:
+
HCl H + Cl -
(aq)
(aq)
(aq)
Consider what happens when hydrogen chloride is mixed into water:
Hydrogen chloride donates a hydrogen ion to one of the
nonbonding electron pairs on a water molecule, resulting in a third
hydrogen bonded to the oxygen. In this case, hydrogen chloride
behaves as an acid (proton donor) and water behaves as a base
(proton acceptor). The products of this reaction are a chloride ion
+
and a hydronium ion, H O , which, as Figure 5 shows, is made by
3
adding a proton (hydrogen ion) to a water molecule.
5