Page 51 - YORAM RUDY BOOK FINAL
P. 51
P. 51
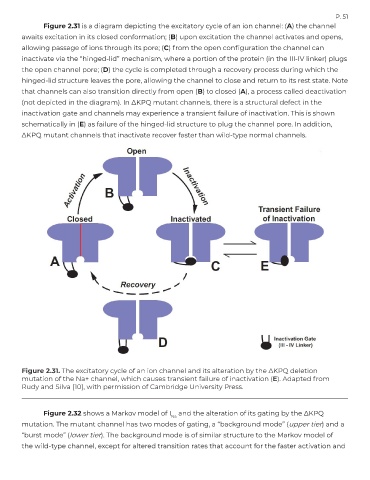
Figure 2.31 is a diagram depicting the excitatory cycle of an ion channel: (A) the channel
awaits excitation in its closed conformation; (B) upon excitation the channel activates and opens,
allowing passage of ions through its pore; (C) from the open configuration the channel can
inactivate via the “hinged-lid” mechanism, where a portion of the protein (in the III-IV linker) plugs
the open channel pore; (D) the cycle is completed through a recovery process during which the
hinged-lid structure leaves the pore, allowing the channel to close and return to its rest state. Note
that channels can also transition directly from open (B) to closed (A), a process called deactivation
(not depicted in the diagram). In ΔKPQ mutant channels, there is a structural defect in the
inactivation gate and channels may experience a transient failure of inactivation. This is shown
schematically in (E) as failure of the hinged-lid structure to plug the channel pore. In addition,
ΔKPQ mutant channels that inactivate recover faster than wild-type normal channels.
Figure 2.31. The excitatory cycle of an ion channel and its alteration by the ∆KPQ deletion
mutation of the Na+ channel, which causes transient failure of inactivation (E). Adapted from
Rudy and Silva [10], with permission of Cambridge University Press.
Figure 2.32 shows a Markov model of I and the alteration of its gating by the ΔKPQ
Na
mutation. The mutant channel has two modes of gating, a “background mode” (upper tier) and a
“burst mode” (lower tier). The background mode is of similar structure to the Markov model of
the wild-type channel, except for altered transition rates that account for the faster activation and