Page 11 - Group 4_Project_CESP2021
P. 11
Based on the explanation above, it can be concluded that a covalent bond is a
bond that occurs due to the sharing of an electron pair by two atoms. A covalent bond
is formed between two atoms that both want to gain electrons. Generally covalent bonds
are formed by non-metallic elements which can be of the same type (e.g. H2, N2, O2,
Cl2, F2, Br2, I2) and different types (e.g. H2O, CO2, etc.). Compounds that contain only
covalent bonds are called covalent compounds. The use of electron pairs in covalent
bonds can be described by Lewis structures.
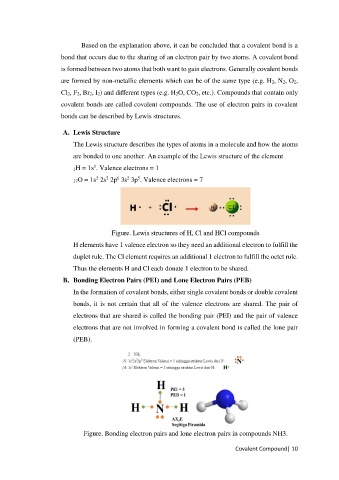
A. Lewis Structure
The Lewis structure describes the types of atoms in a molecule and how the atoms
are bonded to one another. An example of the Lewis structure of the element
1
1H = 1s . Valence electrons = 1
6
2
2
2
5
17O = 1s 2s 2p 3s 3p . Valence electrons = 7
Figure. Lewis structures of H, Cl and HCl compounds
H elements have 1 valence electron so they need an additional electron to fulfill the
duplet rule. The Cl element requires an additional 1 electron to fulfill the octet rule.
Thus the elements H and Cl each donate 1 electron to be shared.
B. Bonding Electron Pairs (PEI) and Lone Electron Pairs (PEB)
In the formation of covalent bonds, either single covalent bonds or double covalent
bonds, it is not certain that all of the valence electrons are shared. The pair of
electrons that are shared is called the bonding pair (PEI) and the pair of valence
electrons that are not involved in forming a covalent bond is called the lone pair
(PEB).
Figure. Bonding electron pairs and lone electron pairs in compounds NH3.
Covalent Compound| 10