Page 486 - The Toxicology of Fishes
P. 486
466 The Toxicology of Fishes
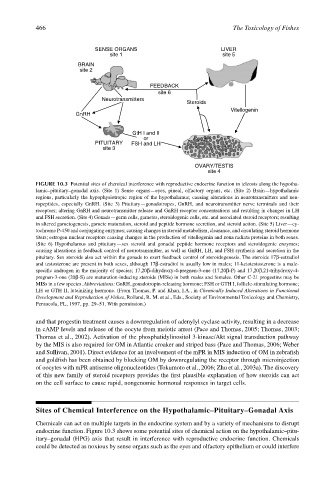
SENSE ORGANS LIVER
site 1 site 5
BRAIN
site 2
FEEDBACK
site 6
Neurotransmitters
Steroids
Vitellogenin
GnRH
GtH I and II
or
PITUITARY FSH and LH
site 3
OVARY/TESTIS
site 4
FIGURE 10.3 Potential sites of chemical interference with reproductive endocrine function in teleosts along the hypotha-
lamic–pituitary–gonadal axis. (Site 1) Sense organs—eyes, pineal, olfactory organs, etc. (Site 2) Brain—hypothalamic
regions, particularly the hypophysiotropic region of the hypothalamus; causing alterations in neurotransmitters and neu-
ropeptides, especially GnRH. (Site 3) Pituitary—gonadotropes, GnRH, and neurotransmitter nerve terminals and their
receptors; altering GnRH and neurotransmitter release and GnRH receptor concentrations and resulting in changes in LH
and FSH secretion. (Site 4) Gonads—germ cells, gametes, steroidogenic cells, etc. and associated steroid receptors; resulting
in altered gametogenesis, gamete maturation, steroid and peptide hormone secretion, and steroid action. (Site 5) Liver—cy-
tochrome P-450 and conjugating enzymes; causing changes in steroid metabolism, clearance, and circulating steroid hormone
titers; estrogen nuclear receptors causing changes in the production of vitellogenin and zona radiata proteins in both sexes.
(Site 6) Hypothalamus and pituitary—sex steroid and gonadal peptide hormone receptors and steroidogenic enzymes;
causing alterations in feedback control of neurotransmitter, as well as GnRH, LH, and FSH synthesis and secretion in the
pituitary. Sex steroids also act within the gonads to exert feedback control of steroidogenesis. The steroids 17β-estradiol
and testosterone are present in both sexes, although 17β-estradiol is usually low in males; 11-ketotestosterone is a male-
specific androgen in the majority of species; 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P) and 17,20β,21-trihydroxy-4-
pregnen-3-one (20β-S) are maturation-inducing steroids (MISs) in both males and females. Other C-21 progestins may be
MISs in a few species. Abbreviations: GnRH, gonadotropin-releasing hormone; FSH or GTH I, follicle-stimulating hormone;
LH or GTH II, luteinizing hormone. (From Thomas, P. and Khan, I.A., in Chemically Induced Alterations in Functional
Development and Reproduction of Fishes, Rolland, R. M. et al., Eds., Society of Environmental Toxicology and Chemistry,
Pensacola, FL, 1997, pp. 29–51. With permission.)
and that progestin treatment causes a downregulation of adenylyl cyclase activity, resulting in a decrease
in cAMP levels and release of the oocyte from meiotic arrest (Pace and Thomas, 2005; Thomas, 2003;
Thomas et al., 2002). Activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway
by the MIS is also required for OM in Atlantic croaker and striped bass (Pace and Thomas, 2006; Weber
and Sullivan, 2001). Direct evidence for an involvement of the mPR in MIS induction of OM in zebrafish
and goldfish has been obtained by blocking OM by downregulating the receptor through microinjection
of oocytes with mPR antisense oligonucleotides (Tokumoto et al., 2006; Zhu et al., 2003a). The discovery
of this new family of steroid receptors provides the first plausible explanation of how steroids can act
on the cell surface to cause rapid, nongenomic hormonal responses in target cells.
Sites of Chemical Interference on the Hypothalamic–Pituitary–Gonadal Axis
Chemicals can act on multiple targets in the endocrine system and by a variety of mechanisms to disrupt
endocrine function. Figure 10.3 shows some potential sites of chemical action on the hypothalamic–pitu-
itary–gonadal (HPG) axis that result in interference with reproductive endocrine function. Chemicals
could be detected as noxious by sense organs such as the eyes and olfactory epithelium or could interfere