Page 491 - The Toxicology of Fishes
P. 491
The Endocrine System 471
OH OH
OH C 9 H 19
HO
HO
HO
17β-Estradiol Diethylstilbestrol p-Nonylphenol
OH
OH O OH O CH 3 H 3 C CH 3
O
HO O HO O HO OH
Genistein Zearalenone Bisphenol-A
Cl
Cl
Cl Cl
Cl Cl Cl
Cl Cl
Cl C Cl Cl
Cl
Cl
CCl 3
Cl Cl
O
o,p'-DDT Kepone 2,2',5'-PCB-4-OH
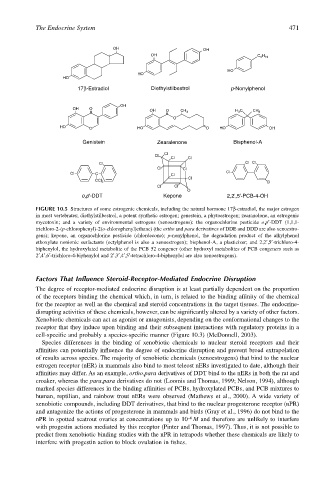
FIGURE 10.5 Structures of some estrogenic chemicals, including the natural hormone 17β-estradiol, the major estrogen
in most vertebrates; diethylstilbestrol, a potent synthetic estrogen; genestein, a phytoestrogen; zearanolone, an estrogenic
mycotoxin; and a variety of environmental estrogens (xenoestrogens): the organochlorine pesticide o,p′-DDT (1,1,1-
trichloro-2-(p-chlorophenyl)-2(o-chlorophenyl)ethane) (the ortho and para derivatives of DDE and DDD are also xenoestro-
gens); kepone, an organochlorine pesticide (chlordecone); p-nonylphenol, the degradation product of the alkylphenol
ethoxylate nonionic surfactants (octylphenol is also a xenoestrogen); bisphenol-A, a plasticizer; and 2,2′,5′-trichloro-4-
biphenylol, the hydroxylated metabolite of the PCB 52 congener (other hydroxyl metabolites of PCB congeners such as
2′,4′,6′-trichloro-4-biphenylol and 2′,3′,4′,5′-tetrachloro-4-biphenylol are also xenoestrogens).
Factors That Influence Steroid-Receptor-Mediated Endocrine Disruption
The degree of receptor-mediated endocrine disruption is at least partially dependent on the proportion
of the receptors binding the chemical which, in turn, is related to the binding affinity of the chemical
for the receptor as well as the chemical and steroid concentrations in the target tissues. The endocrine-
disrupting activities of these chemicals, however, can be significantly altered by a variety of other factors.
Xenobiotic chemicals can act as agonist or antagonists, depending on the conformational changes to the
receptor that they induce upon binding and their subsequent interactions with regulatory proteins in a
cell-specific and probably a species-specific manner (Figure 10.3) (McDonnell, 2003).
Species differences in the binding of xenobiotic chemicals to nuclear steroid receptors and their
affinities can potentially influence the degree of endocrine disruption and prevent broad extrapolation
of results across species. The majority of xenobiotic chemicals (xenoestrogens) that bind to the nuclear
estrogen receptor (nER) in mammals also bind to most teleost nERs investigated to date, although their
affinities may differ. As an example, ortho,para derivatives of DDT bind to the nERs in both the rat and
croaker, whereas the para,para derivatives do not (Loomis and Thomas, 1999; Nelson, 1994), although
marked species differences in the binding affinities of PCBs, hydroxylated PCBs, and PCB mixtures to
human, reptilian, and rainbow trout nERs were observed (Mathews et al., 2000). A wide variety of
xenobiotic compounds, including DDT derivatives, that bind to the nuclear progesterone receptor (nPR)
and antagonize the actions of progesterone in mammals and birds (Gray et al., 1996) do not bind to the
nPR in spotted seatrout ovaries at concentrations up to 10 M and therefore are unlikely to interfere
–4
with progestin actions mediated by this receptor (Pinter and Thomas, 1997). Thus, it is not possible to
predict from xenobiotic binding studies with the nPR in tetrapods whether these chemicals are likely to
interfere with progestin action to block ovulation in fishes.