Page 494 - The Toxicology of Fishes
P. 494
474 The Toxicology of Fishes
120
[ 3 H]-Estradiol bound (%) 80 E2
100
60
o,p'-DDT
o,p'-DDE
40
Nonylphenol
20 2,2',5'-PCB-4-OH
Aroclor 1254
Zerealenone
0
10 –12 10 –11 10 –10 10 –9 10 –8 10 –7 10 –6 10 –5 10 –4 10 –3
Concentration (M)
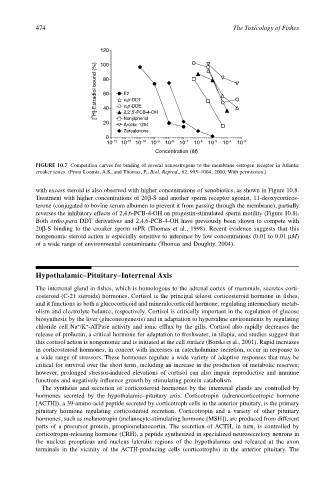
FIGURE 10.7 Competition curves for binding of several xenoestrogens to the membrane estrogen receptor in Atlantic
croaker testes. (From Loomis, A.K. and Thomas, P., Biol. Reprod., 62, 995–1004, 2000. With permission.)
with excess steroid is also observed with higher concentrations of xenobiotics, as shown in Figure 10.8.
Treatment with higher concentrations of 20β-S and another sperm receptor agonist, 11-deoxycorticos-
terone (conjugated to bovine serum albumen to prevent it from passing through the membrane), partially
reverses the inhibitory effects of 2,4,6-PCB-4-OH on progestin-stimulated sperm motility (Figure 10.8).
Both ortho,para DDT derivatives and 2,4,6-PCB-4-OH have previously been shown to compete with
20β-S binding to the croaker sperm mPR (Thomas et al., 1998). Recent evidence suggests that this
nongenomic steroid action is especially sensitive to inference by low concentrations (0.01 to 0.01 µM)
of a wide range of environmental contaminants (Thomas and Doughty, 2004).
Hypothalamic–Pituitary–Interrenal Axis
The interrenal gland in fishes, which is homologous to the adrenal cortex of mammals, secretes corti-
costeroid (C-21 steroids) hormones. Cortisol is the principal teleost corticosteroid hormone in fishes,
and it functions as both a glucocorticoid and mineralocorticoid hormone, regulating intermediary metab-
olism and electrolyte balance, respectively. Cortisol is critically important in the regulation of glucose
biosynthesis by the liver (gluconeogenesis) and in adaptation to hypersaline environments by regulating
+
chloride cell Na /K -ATPase activity and ionic efflux by the gills. Cortisol also rapidly decreases the
+
release of prolactin, a critical hormone for adaptation to freshwater, in tilapia, and studies suggest that
this cortisol action is nongenomic and is initiated at the cell surface (Borski et al., 2001). Rapid increases
in corticosteroid hormones, in concert with increases in catecholamine secretion, occur in response to
a wide range of stressors. These hormones regulate a wide variety of adaptive responses that may be
critical for survival over the short term, including an increase in the production of metabolic reserves;
however, prolonged stressor-induced elevations of cortisol can also impair reproductive and immune
functions and negatively influence growth by stimulating protein catabolism.
The synthesis and secretion of corticosteroid hormones by the interrenal glands are controlled by
hormones secreted by the hypothalamic–pituitary axis. Corticotropin (adrenocorticotropic hormone
[ACTH]), a 39-amino-acid peptide secreted by corticotroph cells in the anterior pituitary, is the primary
pituitary hormone regulating corticosteroid secretion. Corticotropin and a variety of other pituitary
hormones, such as melanotropin (melanocyte-stimulating hormone [MSH]), are produced from different
parts of a precursor protein, proopiomelanocortin. The secretion of ACTH, in turn, is controlled by
corticotropin-releasing hormone (CRH), a peptide synthesized in specialized neurosecretory neurons in
the nucleus preopticus and nucleus lateralis regions of the hypothalamus and released at the axon
terminals in the vicinity of the ACTH-producing cells (corticotrophs) in the anterior pituitary. The